Abstract
Background
The acidic pH of the stratum corneum (SC) is important for epidermal permeability barrier homeostasis. Acidification of the skin surface has been suggested as a therapeutic strategy for skin disorders such as atopic dermatitis (AD).
Objective
We performed an animal study to evaluate the usefulness of acidification of SC for inhibition of AD lesions and to find out if the therapeutic effect of vinegar is attributable to its herbal contents, rather than its acidity.
Methods
Five groups of six oxazolone-treated (Ox)-AD mice were treated for three weeks with creams of different acidity: vehicle cream alone (pH 5.5), neutralized vinegar cream (pH 7.4), pH 5.0 vinegar cream, pH 3.5 vinegar cream, and pH 3.5 hydrogen chloride (HCl) cream. Also, we have compared two groups of Ox-AD mice treated with pH 5.5 vehicle cream or pH 5.5 vinegar cream.
Results
Ox-AD mice treated with acidic creams exhibited fewer AD-like lesions, had significantly lower eczema scores, decreased basal by transepidermal water loss (TEWL), and increased SC hydration compared to the groups given only vehicle and neutral cream. There was no significant difference between the acidic vinegar and HCl groups. Between the groups treated with vehicle and pH 5.5 vinegar cream, there was no difference in eczema score, basal TEWL and SC hydration.
The stratum corneum (SC) of skin normally has an acidic pH, which is referred to as the “acid mantle”1. This acidic pH is important for the protective functions of skin, including permeability barrier homeostasis123, SC integrity and cohesion13, antimicrobial defense1456, and primary cytokine activation7. Three endogenous pathways as well as exogenous mechanisms have been identified as contributing to the acidic pH of the SC: 1) the non-energy-dependent Na+/H+ antiporter, NHE18, 2) generation of free fatty acids from phospholipids by secretory phospholipase A23910, and 3) generation of urocanic acid from histidine by histidase11. Deterioration of any of these pathways leads to an elevation in SC pH linked to the alteration of permeability barrier homeostasis and SC integrity/ cohesion.
The pH of the SC increases in developmentally impaired neonatal skin, aged skin121314, and inflammed skin15 such as that found in atopic dermatitis (AD). Therefore, the reversal of pH abnormality may be a possible preventive or therapeutic strategy for these problems1617. Acidification of the SC improves permeability barrier homeostasis by increasing the activity of the two key ceramide-generating enzymes such as β-glucocerebrosidase and acidic sphingomyelinase131819, and reinforces SC integrity and cohesion by decreasing the activity of serine proteases (SPs). SPs not only inactivate lipid-processing enzymes18, but also degrade the proteins that form corneodesomsomes1 and inhibit the secretion of lamellar body20.
In the oxazolone-induced AD (Ox-AD) murine model, acidification of the SC by topical application of lactobionic acid as polyhydroxyl acid prevented the emergence of the features of AD by maintaining normal barrier function and resulted in a significant reduction in histologic evidence of inflammation16. Recently we also reported that acidic cream inhibited the occurrence of respiratory allergic inflammation as well as AD-like skin lesions in atopic march murine models developed in flaky tail mice and Ox-AD mice by repeated house dust mite application2122. Clinically, a therapeutic effect of acidic water bathing on AD patients was also evaluated by us23.
Vinegar has been traditionally used in Korean folk medicine for the treatment of many dermatoses including eczema. The therapeutic effect has been assumed to be attributable to various source materials such as herbal ingredients contained in the vinegar.
To find out if the therapeutic effect of vinegar is attributable to its source materials, rather than its acidity, we conducted an animal experiment using Ox-AD mice treated with creams of different acidity made using different sources of acid including Korean traditional vinegar.
All animal procedures were approved by the Yonsei University at Wonju College of Medicine Institutional Animal Care and Use Committee (2011-49). Thirty female hairless mice (hr/hr) were obtained from OrientBio (Seongnam, Korea) and Ox was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Cetaphil® cream (Galderma Laboratories, Cranbury, NJ, USA) was used as the vehicle.
To generate mice with AD-like dermatitis, all mice were sensitized with a single topical application of 1% Ox. After one week of Ox sensitization, the mice were treated topically with 0.1% Ox once every other day for 3 weeks2425. Ox-AD mice were divided into five groups of 6 mice each, depending on the creams applied to their skin. Mice were treated with vehicle cream alone (pH 5.5, Cetaphil®), neutralized vinegar cream (pH 7.4), pH 5.0 vinegar cream, pH 3.5 vinegar cream, or pH 3.5 hydrochloric acid (HCl) cream twice daily for 3 weeks, in parallel with Ox challenges. All of the acidic creams were made in our laboratory using Cetaphil® cream as vehicle and vinegar. Vinegar was added to the vehicle cream until the pH reaches pH 3.5. To make pH 5.0 or 5.5 vinegar creams, 10mM NaOH was also added until pH rises up to the levels we intended. Vinegar is a fermented acid originated from fruits and herbs home made by traditional method which contains various acetic acids, amino acids and many herbal contents that are thought to have anti-oxidative effects in oriental medicine from ancient times. The creams were applied 1 hour after Ox challenges on the day both interventions are done. Skin surface pH of normal young mice is around pH 5.514, and the pH of vehicle (Cetaphil®) cream itself was 5.5. We wanted to figure out if the herbal contents in vinegar have a beneficial effect on skin permeability barrier function, other than acidity. So, we have done an additional experiment using Ox-AD mice to compare the vehicle and vinegar cream with same acidity (pH 5.5). The application of vehicle (Cetaphil® cream) and pH 5.5 vinegar cream was done to two groups of mice, four mice each as described previously.
Eczema scores were measured and photographs were taken of gross AD lesions of each group before and after the application of topical creams. The eczema clinical score was defined as the sum of individual scores graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for the symptoms of erythema, edema, and lichenification2627. The excoriation was excluded as it is difficult to be evaluated from mice subjects. Transepidermal water loss (TEWL) was measured on the dorsal skin of mice using a Tewameter (Courage & Khazaka, Cologne, Germany), and SC hydration was measured with a Corneometer (Courage & Khazaka) before and after the application of topical creams.
To estimate any side effects with acidic creams, we applied acidic creams (pH 5.0 vinegar, pH 3.5 vinegar, and pH 3.5 HCl creams) on normal skin of hairless mice for 3 weeks twice daily without any additional interventions.
To evaluate how long the lowered pH by application of acidic creams maintains, we performed a test on normal human skin. As the measurement of skin surface pH can be influenced by psychological stress and physical activity of mice, the test could not be done with animals. Therefore, we applied acidic creams (pH 3.5 vinegar, pH 3.5 HCl, and pH 5.0 vinegar cream) on human forearms with normal skin (n=4). Skin surface pH was measured right after application of acidic creams, 15 minutes, 1 hour, 3 hours, 6 hours, and 12 hours after application. The experiment was done only once.
To compare eczema scores, basal TEWL, and SC hydration in Ox-AD mice groups, we used the one-way ANOVA test. Dunnett test for the post test was done to compare each group with the vehicle group. Statistical analyses were performed using Prism 5 (Graphad Software, San Diego, CA, USA).
Initial Ox challenge provoked acute allergic contact dermatitis, and multiple Ox challenges resulted in the development of AD-like lesions. To determine whether SC acidification by application of acidic cream including vinegar prevents the development of AD-like lesions, we compared the gross lesions of the Ox-AD mice in different treatment groups. Compared to the mice groups given only vehicle cream, the mice groups given the acidic pH 5.0 vinegar cream, pH 3.5 vinegar cream, or pH 3.5 HCl cream exhibited fewer AD-like lesions with less erythema, edema, scales, and lichenification. There was no difference between mice group given only vehicle cream and those given neutralized (pH 7.4) vinegar cream (Fig. 1).
Repeated Ox challenges producing AD-like lesions disrupted the epidermal permeability barrier in mice. To evaluate the effect of SC acidification including vinegar therapy on skin barrier function, we compared basal TEWL between groups. The basal TEWLs of the vehicle cream (Cetaphill® cream, pH 5.5) and neutralized vinegar cream (pH 7.4) groups were significantly higher than those of the pH 5.0 vinegar, pH 3.5 vinegar, and pH 3.5 HCl groups (Fig. 2A). Likewise, SC hydration, which is known to be low in AD skin, was significantly lower in the vehicle and neutralized vinegar groups than in the pH 5.0 vinegar, pH 3.5 vinegar, and pH 3.5 HCl groups (Fig. 2B). These results imply that SC acidification, not just from vinegar treatment, can improve the epidermal permeability barrier disrupted due to Ox treatment. In the additional experiment, there was no difference in clinical eczema score, basal TEWL, SC hydration between the vehicle group (pH 5.5) and the pH 5.5 vinegar group (Fig. 3). By this we could regard the effect of vinegar on epidermal permeability barrier results from its acidic pH, not due to its herbal contents. After applying acidic creams on normal skin of hairless mice for 3 weeks, no mouse showed scratching behavior or abnormal skin finding, implying that acidic creams have no cutaneous side effects.
Right after application of acidic creams, the skin surface pH immediately decreased except for pH 5.0 vinegar cream. Although pH rised after 15 minutes, the slope of increase was gentle and low pH maintained throughout 6 hours after application of creams (Fig. 4).
The acidic pH of the SC is critical for key epidermal functions such as permeability barrier homeostasis, SC integrity/cohesion (desquamation), initiation of skin inflammation, and antimicrobial defense28. In inflammatory dermatoses, both an elevation in the SC pH and deterioration of these key functions are observed.
Recently, the efficacy of SC acidification in the treatment of developmentally impaired skin has been studied. Hyper-acidification was revealed to enhance the structure and function of normal mouse skin17 and to normalize the impaired barrier function of neonatal29 and aged14 human and in rodent skin in which SC acidification is impaired. It was demonstrated that maintenance of SC acidity by topical acidic application prevents the emergence of AD16 and the occurrence of respiratory allergic inflammation. Our recent study22 was performed to evaluate if the development of airway allergy as well as AD can be prevented by acidification of skin with fixed pH (2.8) cream by using atopic march model, whereas this study was to find out if AD development can be different according to applied pH in AD model.
Traditional vinegar, which is extracted from fermented herbs, has long been used for the treatment of some dermatitis including eczema in Korean folk medicine. We believe that its efficacy for the treatment of eczema was due to its acidity, not due to the herbal content. Therefore, in order to elucidate whether the effects of traditional vinegar treatment result from its acidity or from other components of source materials, we compared the effects of treatment with pH 3.5 or 5.0 vinegar cream to pH 3.5 HCl and pH 5.5 vehicle cream in an Ox-AD murine model. Ox-AD mice treated with low pH creams showed less AD-like lesions and better epidermal permeability barrier function compared to those treated with vehicle cream or neutralized cream. On the other hand, Ox-AD mice treated with pH 5.5 vinegar cream showed the same permeability barrier functions and AD lesions as vehicle (pH 5.5) group. This finding suggests that pH 5.5 is not enough to improve skin barrier in Ox-AD murine model and the other ingredients of the vinegar do not contribute to improved skin barrier function and anti-inflammation. The two experiments described above were preformed separately, using different mice groups. Therefore, the values of eczema scores, TEWL, and SC hydration may be quite different in the subjects.
We have already done an experiment using topical corticosteroid in other study2230. Through the results of these both experiments, we have confirmed that acidification of skin had comparable effect for skin permeability barrier as topical corticosteroid, so we did not use positive control in this study.
In our additional study to evaluate the maintenance of acidic pH of skin surface obtained by acidic creams, the pH dropped immediately after application and low pH tended to rise slowly but maintained throughout 6 hours except for pH 5.0 vinegar or vehicle cream. On the other hand, the Ox-AD mice group given the pH 5.0 vinegar cream exhibited fewer AD-like lesions and improved skin permeability barrier functions than the vehicle group (pH 5.5). We believe that the discrepancy owes to the difference in the thickness of SC layer between human and mouse skin. As the SC of mouse skin is much thinner than human, the acidic cream could pass through the SC more easily and quickly. Furthermore, disrupted skin barrier of Ox-AD mouse skin would have assisted for the penetration of acidic creams into the SC-SG junction to take effect.
In addition, NaOH added to neutralize the cream may have acted as an irritant and caused bias the results. However, according to Fig. 1 and Fig. 2, there was no statistical difference in eczema score and skin barrier function between pH 3.5 and pH 5.0 vinegar cream applied groups. If NaOH acted as an irritant and vinegar itself acted to protect skin barrier, then pH 3.5 vinegar cream applied group should have shown a better skin barrier function, which did not. Therefore this implies that in the setting of low pH, the NaOH does not act as an irritant. This corresponds with the report by Korting et al.31, which showed no difference in skin roughness and TEWL that represent skin irritation, when the same cleansing preparations except for the pH itself based on a different amount of NaOH included are used. In Fig. 4, the pH showed to be elevated temporarily after application of pH 5.0 vinegar cream, but statistically insignificant. The beneficial effect on skin permeability barrier of acid application has been already well described in a review article by Ali and Yosipovitch32, twice daily application of lactic acid formulation significantly improved barrier function as measured by TEWL and increased total ceramide fraction in vivo. Therefore, we concluded that the acidic cream application showed beneficial effect on skin barrier function solely due to its acidity.
Our study shows again that acidification of the SC inhibits the development of AD lesions in Ox-AD mice through improvement of skin barrier function regardless of the source materials of the acid. Also, we could identify that vinegar renders beneficial effects on AD due to its acidity, not by its herbal contents. Collectively, maintenance of acidic skin surface pH by topical application of acidic cream could be an effective therapeutic modality for AD by improvement of skin barrier function.
Figures and Tables
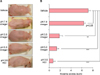 | Fig. 1(A) Oxazolone-treated atopic dermatitis (Ox-AD) mice groups treated with pH 3.5 vinegar cream, pH 5.0 vinegar cream, or pH 3.5 hydrogen chloride (HCl) cream exhibited fewer AD-like lesions than mice treated with only vehicle cream (pH 5.5) or neutralized vinegar cream (pH 7.4). (B) Eczema scores were significantly higher in the vehicle group than in the acidic cream treatment groups. There was no difference in the eczema scores between vehicle group and neutralized vinegar cream treated group. Eczema scores were determined by the sum of the severity of erythema, edema, and lichenification. Severity: absent (0), mild (1), moderate (2), and severe (3). Results are shown as the mean±standard error of the mean. **p<0.05, ***p<0.001. |
 | Fig. 2(A, B) Significant decreases in basal transepidermal water loss (TEWL) and significant increases in stratum corneum (SC) hydration were observed in the mouse groups treated with pH 3.5 hydrogen chloride (HCl) cream or pH 3.5 and 5.0 vinegar creams compared with vehicle (veh.) or neutralized vinegar (neut vin.) cream. No significant differences were seen between the acidic vinegar-treated and HCl-treated group. Results are shown as the mean±standard error of the mean. |
 | Fig. 3(A) Eczema scores and (B) skin permeability barrier functions of Ox induced AD model mice after application of vehicle and pH 5.5 vinegar creams. Ox: oxazolone-treated, AD: atopic dermatitis, TEWL: transepidermal water loss, SC: stratum corneum. |
 | Fig. 4Maintenance of acidic pH after application of pH 3.5 vinegar, pH 3.5 hydrogen chloride (HCl) and pH 5.0 vinegar cream. Immediately after application of acidic cream, the skin surface pH decreased in pH 3.5 vinegar cream and HCl cream but not in pH 5.0 vinegar cream. Although pH rised after 15 minutes, the slope of increase was gentle, therefore low pH was maintained throughout 6 hours after application of acidic creams. |
ACKNOWLEDGMENT
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant no. HN10C0033).
References
1. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003; 121:345–353.

2. Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998; 290:215–222.

3. Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001; 117:44–51.

4. Devillers AC, de Waard-van der Spek FB, Mulder PG, Oranje AP. Treatment of refractory atopic dermatitis using ‘wet-wrap’ dressings and diluted corticosteroids: results of standardized treatment in both children and adults. Dermatology. 2002; 204:50–55.

Korting HC., Hübner K., Greiner K., Hamm G., Braun-Falco O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of a crossover trial in healthy volunteers. Acta Derm Venereol. 1990. 70:429–431.
7. Hachem JP, Behne M, Fluhr J, Feingold KR, Elias PM. Increased stratum corneum pH promotes activation and release of primary cytokine from the stratum corneum attributable to activation of serine proteases. J Invest Dermatol. 2002; 119:258.
8. Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, et al. NHE regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002; 277:47399–47406.
9. Mao-Qiang M, Jain M, Feingold KR, Elias PM. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J Invest Dermatol. 1996; 106:57–63.

10. Mazereeuw-Hautier J, Redoules D, Tarroux R, Charveron M, Salles JP, Simon MF, et al. Identification of pancreatic type I secreted phospholipase A2 in human epidermis and its determination by tape stripping. Br J Dermatol. 2000; 142:424–431.

11. Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum -an unexpected role for urocanic acid. J Invest Dermatol. 2000; 115:414–420.

12. Behne MJ, Barry NP, Hanson KM, Aronchik I, Clegg RW, Gratton E, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003; 120:998–1006.

13. Fluhr JW, Behne MJ, Brown BE, Moskowitz DG, Selden C, Mao-Qiang M, et al. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004; 122:320–329.

14. Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007; 127:2847–2856.

15. Fluhr JW, Elias PM. Stratum corneum pH: formation and function of the ‘acid mantle’. Exog Dermatol. 2002; 1:163–175.

16. Hatano Y, Man MQ, Uchida Y, Crumrine D, Scharschmidt TC, Kim EG, et al. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J Invest Dermatol. 2009; 129:1824–1835.

17. Hachem JP, Roelandt T, Schürer N, Pu X, Fluhr J, Giddelo C, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2010; 130:500–510.

18. Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005; 125:510–520.

19. Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006; 580:5456–5466.

20. Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006; 126:2074–2086.

21. Lee HJ, Lee NR, Jung M, Kim DH, Choi EH. Atopic march from atopic dermatitis to asthma-like lesions in NC/Nga mice is accelerated or aggravated by neutralization of stratum corneum but partially inhibited by acidification. J Invest Dermatol. 2015; 135:3025–3033.

22. Lee HJ, Yoon NY, Lee NR, Jung M, Kim DH, Choi EH. Topical acidic cream prevents the development of atopic dermatitis- and asthma-like lesions in murine model. Exp Dermatol. 2014; 23:736–741.

23. Lee NR, Lee HJ, Yoon NY, Kim D, Jung M, Choi EH. Acidic water bathing could be a safe and effective therapeutic modality for severe and refractory atopic dermatitis. Ann Dermatol. 2016; 28:126–129.

24. Lee HJ, Jung M, Kim JH, Yoon NY, Choi EH. The effect of adipose-derived stem cell-cultured media on oxazolone treated atopic dermatitis-like murine model. Ann Dermatol. 2012; 24:181–188.

25. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008; 128:79–86.

26. Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, et al. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999; 120:Suppl 1. 70–75.

27. Yang F, Tanaka M, Wataya-Kaneda M, Yang L, Nakamura A, Matsumoto S, et al. Topical application of rapamycin ointment ameliorates Dermatophagoides farina body extract-induced atopic dermatitis in NC/Nga mice. Exp Dermatol. 2014; 23:568–572.

28. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005; 125:183–200.

29. Fluhr JW, Mao-Qiang M, Brown BE, Hachem JP, Moskowitz DG, Demerjian M, et al. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 2004; 123:140–151.

30. Yoon NY, Jung My, Kim DH, Lee HJ, Choi EH. Topical glucocorticoid or pimecrolimus treatment suppresses thymic stromal lymphopoietin-related allergic inflammatory mechanism in an oxazolone-induced atopic dermatitis murine model. Arch Dermatol Res. 2015; 307:569–581.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download