Dear Editor:
The dermal papilla (DP), a specialized mesenchymal component situated at the base of hair follicles, is thought to play a key role in controlling hair follicle development, hair growth, and hair-cyclic activity1. In experimental trials of hair regeneration, however, two-dimensional (2D)-cultured DP cells have been shown to lose their hair-inductive capacity (trichogenicity) during subculture2. Attempts have therefore been made to maintain trichogenicity of 2D-cultured DP cells by using a wide variety of methods, including supplementation with necessary factors as well as spheroid culturing3. Indeed, recent studies have shown that the trichogenicity of cultured human DP cells is markedly improved by the use of 3D-cultured cells (spheres) rather than 2D-cultured cells4.
Here, we attempted to find a readily available mesenchymal cell source which can substitute for the role of trichogenic DP cells. Since adipose tissue contains a type of adult stem cells originating from the mesenchyme5, we speculated on the potential role of adipocyte precursor cells or stem cells derived from adipose tissues. While the largest depot of adipose tissue is the abdominal subcutaneous adipose tissue under the skin, adipose tissue also exists within non-abdominal locations associated with the skin dermis. This adipose compartment in the dermis underlying reticular dermis, of which development is independent from that of subcutaneous adipose tissue, is defined as the intradermal adipose tissue6. Given that dermal fibroblasts and intradermal adipocytes share a common precursor7, it will be interesting to explore whether intradermal adipocyte precursor cells, if any, in adult skin might serve as the best trichogenic dermal component. However, it is hard to obtain sufficient number of intradermal adipocyte precursor cells for the application to hair induction experiments. On the other hand, adipose-derived stem cells (ADSCs) are easily harvestable, relatively prevalent, and can be isolated from the abdominal fat which is the most easily harvestable and general form of adipose tissues during liposuction. In this study, we, threrefore, investigated whether ADSCs could substitute trichogenic DP cells.
Human DP samples were isolated from hair follicles of non-balding scalp specimens obtained from patients undergoing hair transplantation surgery. DP cells were expanded in 2D culture as described previously8. The Medical Ethical Committee of the Kyungpook National University Hospital (Daegu, Korea) approved all of the described studies (KNUH 2013-02-001-001). Informed written consent was obtained from the patients. Human adipose tissue was obtained from the abdominal fat of one male donor (age: 73 years) and two female donors (age: 43 and 57 years) during surgical operations. Human ADSCs were isolated from the adipose tissue and expanded in 2D culture as described previously9. Cultured DP cells were harvested and seeded (104 cells) into one well of a 96-well hydrocell plate (Nunc, Rochester, NY, USA) to induce the formation of one DP sphere as previously described4. Parallel experiments were performed using ADSCs to form spheroids in the same manner. Seeded plates were incubated at 37℃ in a humidified atmosphere with 5% CO2 for 24~48 h until they were used for implantation. Hair-inductive capacity of human DP and ADSC spheres was assessed as described previously410. Three weeks later, skin samples were excised from the mice and examined to verify hair induction.
As expected, hair follicle formation was observed in positive control experiments in which freshly isolated dermal cells and epidermal cells from the dorsal skin of C57BL/6 mouse neonates at postnatal day 0 (P0) were implanted together (Fig. 1A). No hair induction, however, was observed in experiments in which 2D-cultured human ADSCs were implanted alone (Fig. 1B). Hair follicle formation was also not observed when 2D-cultured human ADSCs (passages two and three) were mixed with newborn mouse epidermal cells (Fig. 1C). Disappointingly, only one to three hair follicles were observed in each cell injection site when ADSC spheres were injected together with mouse epidermal cells (Fig. 1D). There seemed to be no difference of the hair inductive ability among the cell sources from 3 donors of different age and sex. ADSC spheres from female donor aged 43 induced 3 hair follicles, ADSC spheres from male aged 73 induced 2 hair follicles and ADSC spheres from female aged 57 induced 1 hair follicle. However, it is not certain whether the DP cells of newly formed hair follicles would be differentiated from ADSCs. Since there is no panniculus carnosus layer which separates subcutaneous tissue form intradermal adipose tissue, we do not exclude the possibility that there could be some inadvertent inclusion of intradermal fatty tissue or partial contaminant of DP into abdominal subcutaneous adipose tissues during surgical liposuction. In a parallel experiment, reproducible hair induction was observed when DP spheres were implanted together with mouse epidermal cells (Fig. 1F), while no hair induction was observed in experiments in which 2D-cultured human DP cells were combined with mouse epidermal cells before implantation (Fig. 1E). The hair reconstitution assay results are summarized in Table 1.
We next addressed the question of why ADSC spheres showed an extremely low level of hair induction, far below that of the assay using DP spheres. We have examined genes which are considered markers of trichogenic DP cells3, such as versican and Sox2, and compared the expression pattern of those genes between ADSC and DP spheres. We found that DP spheres more abundantly expressed, in terms of mRNA level, versican and Sox2 than the ADSC spheres (Fig. 1G). This real-time PCR analysis indicated that high expression of versican and Sox2 is required for hair-inductive interactions with mouse epidermal cells. Furthermore, the results of the immunochemical analysis showed that DP spheres more abundantly expressed versican protein than the ADSC spheres (Fig. 1H), indicating that high expression of versican is closely correlated with the trichogenicity of dermal mesenchymal components.
In summary, our results show that ADSC spheres possess poor capability to induce hair follicles in contrast to DP spheres. We thus demonstrate in this study that human ADSCs are not a promising option for use as trichogenic dermal cell components. To expand the use of ADSCs to hair folliculogenesis, it is necessary to develop culturing conditions that confer the characteristics of trichogenic DP cells to ADSCs.
Figures and Tables
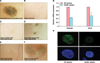 | Fig. 1Hair reconstitution assay and trichogene expression using human adipose-derived stem cells (ADSCs) and dermal papilla (DP) cells. (A) Hair induction was observed in a positive control experiment in which freshly isolated mouse dermal cells (106 cells) and epidermal cells (106 cells) were co-transplanted subcutaneously into the skin on the backs of nude mice. (B) No hair induction was observed when human ADSCs alone (106 cells) were implanted. Two-dimensional (2D)-cultured ADSCs (C) or ADSC spheres (100 in total) grown using hydrocell plates (D) were implanted together with fresh mouse epidermal cells (106 cells). As a control experiment, 2D-cultured DP cells (E) or DP spheres (100 in total) grown using hydrocell plates (F) were implanted together with fresh mouse epidermal cells (106 cells). (G) The mRNA expression of versican and Sox2 in DP spheres was compared to ADSC spheres by real-time PCR analysis. Data are means±standard deviation of triplicates per experiment from two independent experiments. The sequences of primers used in this study are as follows: Versican, Qiagen predesigned primer QT00064064; Sox2, 5'-TTTAGGACAGTTGCAAACGTGAA-3' and 5'-TCAACCTGCATGGCCATTTT-3'; GAPDH, 5'-TGGAAATCCCATCACCATCTTC-3' and 5'-CGCCCCACTTGATTTTGG-3'. (H) Immunofluorescence staining of versican (green) and the corresponding 4', 6-diamidino-2-phenylindole (DAPI) nuclear staining (blue) in DP spheres compared to ADSC spheres. Briefly, the sections were incubated with a mouse monoclonal antibody to versican (1:1,000 dilution; Seikagaku Corporation, Tokyo, Japan), washed with phosphate buffered saline, and incubated with Alexa Flour 488 labeled donkey anti-mouse secondary antibody (1:1,000 dilution; Molecular Probes, Eugene, OR, USA). The slides were then counterstained with DAPI. |
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2015R1A2A2A11000897).
References
1. Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011; 124:1179–1182.

2. Ohyama M, Zheng Y, Paus R, Stenn KS. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol. 2010; 19:89–99.

3. Ohyama M, Veraitch O. Strategies to enhance epithelialmesenchymal interactions for human hair follicle bioengineering. J Dermatol Sci. 2013; 70:78–87.

4. Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012; 132:237–239.

5. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissuederived stem cells. Keio J Med. 2005; 54:132–141.
6. Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014; 23:629–631.

7. Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013; 504:277–281.

8. Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008; 128:262–269.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download