Abstract
Objective
This study investigated the efficacy of three chemical treatments used in humans for hair loss, using a rat model of hair regrowth. The products tested were 2% minoxidil, Hairgrow (Dar-Al-Dawa Pharma), Aminexil, Dercos (Vichy Laboratoires), and Kerium, Anti-chute (La Roche-Posay).
Methods
Thirty-two adult female Wistar-Bratislava rats were assigned to 4 groups. Two rectangular areas (2×4 cm) were shaved on either sides of the mid dorsal line (left side - control; right side - test area). Group I was treated topically with 2% minoxidil, group II with Aminexil, and group III with Kerium. Each rat received 0.3 ml of substance applied topically to the shaved dorsal skin every day for 28 days. Rats in group IV served as sham controls receiving no treatment. Hair regrowth was evaluated by trichoscopy (with a dermatoscope), grown hair weight (from a surface area of 1 cm2), and histopathological examination for skin thickness, follicle count, and percentage of anagen induction (morphometric assessment).
Results
Treatment with 2% minoxidil significantly induced hair regrowth as assessed by trichoscopy, hair weight examination, and morphometric evaluation. Hair weight examination and morphometric assessment demonstrated the lowest hair growth effect with Aminexil among the tested products. Treatment with Kerium was found to significantly induce hair regrowth (p<0.05 as compared to the control group).
Society places considerable emphasis on external appearance, of which, hair is an important component. Scientists and dermatologists claim that hair has an important contribution to the overall appearance of the human body12. Hair loss, although a natural part of the aging process, is often a cause for concern. The profound symbolic and psychosocial importance attached to hair is reflected by the anxiety and distress resulting from its loss3. Cash and colleagues4 assessed the psychological effects of hair loss on women by comparing them with balding men and female control subjects. They confirmed the potentially adverse psychosocial sequelae of this common dermatological condition.
Hair loss and thinning continue to remain common dermatological complaints despite the introduction of many treatment alternatives. Research, aimed at new therapies to prevent hair loss and enhance hair growth, is important as available treatments for alopecia are limited in number as well as in their efficacy4.
The current interest in hair growth promoting agents comes from the perspectives of dermatologists, scientists as well as pharmacological companies5. Experimental animal models such as mice, rats, rabbits, sheep and monkeys have been used to evaluate the extent of hair growth6789. The mouse model, such as the C3H/HeJ mouse, is one of the most widely used animal model reported for testing hair growth products, despite the disadvantages presented by its higher hair density and wave pattern of hair cycle progression101112. Cotsarelis13 demonstrated that in a given person, both the bald areas and the normal scalp had the same number of stem cells. It has also been reported that hair follicles of the alopecic scalp lack progenitor cells. This implies a defect in the activation of stem cells to progenitor cells in the bald scalp. It is known that hair follicles do not disappear in alopecia, they just decrease in size. In order to generate terminal hair, researchers have emphasized the need to identify factors that, when used topically, may convert stem cells into progenitor cells 13.
Results from previous studies have encouraged us to develop our own model for assessing topical therapy for hair regrowth by using healthy adult white Wistar rats714. The purpose of the current study was to compare the efficacy of three chemical treatments used in hair loss patients, namely, 2% minoxidil, Hairgrow (Dar Al Dawa Pharma, Amman, Jordan), Aminexil, Dercos (Vichy Laboratoires, Asnières-sur-Seine, France), and Kerium, Anti-chute (La Roche-Posay, Madrid, Spain). We aimed at determining the differences in hair regrowth induced by the three hair promoting agents in a rat model and to assess the results of treatment by using trichoscopy, hair weight examination, and morphometry, to identify the product with the best results from among the three.
Thirty-two adult female Wistar-Bratislava albino rats (of ages 122~126 days, weighing 200~250 g) were quarantined for 1 week. The animals were held in cages in the Animal Facility of the Physiology Department (4 rats per cage) and maintained on a standard laboratory diet and water ad libitum. They were housed in a room under controlled temperature, with 12 hour light-dark cycles for a minimum of 7 days prior to the experiment.
The study protocol was submitted to and approved by the Institutional Animal Ethical Committee (IAEC) of Iuliu Haţieganu University of Medicine and Pharmacy in Cluj-Napoca, Romania (No. 695/07.02.2013). All experiments were carried in accordance with the Iuliu Haţieganu University of Medicine and Pharmacy guidelines. All experiments were performed in triplicate.
The rats in all groups were preselected to be in the telogen (resting) phase of hair growth cycle, based on their age15. They were randomly assigned to four groups, with each group consisting of eight rats. Prior to shaving the rats, general anesthesia was administered with a combination of ketamine (intraperitoneal 50 mg/kg body weight) and xylazine (20 mg/kg body weight). On the first day of the experiment, fur from two rectangular areas (2×4 cm each), situated on either sides of the spine was removed by using an animal shaving machine. Shaved area on the left side of the spine was defined as the control area was left untreated. The right side was defined as the test area for application of products to be tested.
The animals were divided into four experimental groups (Table 1). Rats in groups I to III received 0.3 ml of hair promoting agent (product) applied topically for 28 days. A syringe plunger was used to apply the product on the test area. Animals were isolated for half an hour after topical application and then housed back to their cages. The rats in the last group (IV) served as control subjects and received no treatment during the experiment. On day 29, the animals were sacrificed by cervical dislocation and then evaluated.
The assessment of hair regrowth efficacy was performed for both the test and control area on day 0 and day 28. The protocol included sedation of the test rats, trichoscopic visualization of the test and control area, measurement of the weight of removed hair (1 cm2 area) and skin biopsy for histological examination. Skin thickness, follicle count, and percentage (%) of anagen (growing phase of the hair cycle) induction were studied for morphometry.
Consistency in using the same camera and settings was maintained in examining animals for trichoscopy. Trichoscopy was performed with DermLite100 dermatoscope (3Gen Inc., San Juan Capistrano, CA, USA) An analytical balance was used for weighing the hair samples. Biopsy sampling was performed by the classical surgical method and not by punch-biopsy technique. Comparisons were performed between the test and control group, as well as between the test and control area for each animal. This allowed each rat to be used as its own control.
This was the first step of evaluation and involved the use of a 4-type scoring system developed by the authors. This included the development of a rating scale of hair regrowth for each Wistar rat, interpreted with reference to the control area of the same animal. Results were reported as: type 1, uneven hair growth on the test area; type 2, low hair density (the skin can be easily seen); type 3, moderate hair density (the skin cannot be seen); type 4, high hair density (full, thick fur).
The second step of hair regrowth evaluation involved measuring the weight of the hair removed from a surface area of 1 cm2 by using an analytical balance. The collected hair was placed on an aluminum foil and labeled to identify the hair as belonging to test or control area. Weight measurement was performed in the chemistry laboratory of the Department of Physiology. Hair weight was measured in milligrams (mg) and expressed as mg/cm2.
Skin biopsies were sampled from both the test and the control areas of rats in all groups. The skin biopsies were immediately fixed in 10% neutral buffered formalin (Chempur; Piekary Śląskie, Poland) and retained for at least 24 hour before processing for standard histopathological examination. Serial sections of 5 µm were sliced and stained with hematoxylin and eosin. Follicles were counted manually in all layers of skin by an observer blinded to the experiment. Skin thickness from epidermis to panniculus carnosus was measured by using conventional light microscopy (Olympus BX 51 [Olympus, Tokyo, Japan] microscope equipped with Olympus SP 350 [Olympus] digital camera and Cell B image analysis software). The percentage of anagen induction was calculated by using the following formula: (follicles in subcutaneous layer) ×100/(total follicle count).
All values were expressed as mean±standard error of mean. Results of trichoscopy evaluation and hair weight measurement were analyzed by Student's t-test. All data used for the statistical analysis were found to be normally distributed (Shapiro-Wilk normality test). Statistical analysis of morphometry data was performed by using two-sample t-test. A value of p<0.05 was considered to be statistically significant. The Statistical Package for the Social Sciences 16.0 for Windows (SPSS Inc., Chicago, IL, USA) and R' software were used for statistical analysis.
A general clinical evaluation of all animals was performed on day 28, at the end of the experimental period (Fig. 1). Treatment with 2% minoxidil was considered as the gold standard and the in vivo hair regrowth induced by it was compared with the results achieved with Aminexil and Kerium therapy in addition to the comparisons involving control areas and the control group. The hair regrowth noticed in group II (Aminexil) and group III (Kerium) was lesser than that seen in groups I (minoxidil) or IV (controls). When evaluated with trichoscopy, the rats from these two groups had type 1 pattern of hair growth (uneven hair growth on tested area) and type 2 pattern of hair growth (low hair density) (Fig. 2, 3). Additionally, both Aminexil and Kerium treated areas had a lower hair regrowth effect when compared to control group (group IV).
Rats in group I (minoxidil) had increased hair growth compared to the control group (Fig. 3). Rats in group I had better hair regrowth than the rats in groups II (Aminexil) and III (Kerium) (Fig. 2, 3). This confirms that 2% minoxidil is more efficacious than Aminexil and Kerium.
The best hair regrowth in the four groups was observed in group I (2% minoxidil). Seven out of 8 rats in group I demonstrated type 3 or type 4 pattern of hair growth (moderate to high hair density). The treatment effects in terms of hair regrowth in group I were significantly better than the hair regrowth observed in the control group (Fig. 2, 3).
The weight of newly grown hair in all the test groups was assessed and compared with that from the control site (Table 2). Hair weight from test areas was significantly higher than from the negative control area when compared in the same animal in group I (minoxidil) and group II (Kerium) (p<0.05). The highest hair weight was recorded in the Kerium treated rats. The hair regrowth stimulation with 2% minoxidil was significantly better than the hair regrowth in negative controls (group IV) (p<0.001). Aminexil topical treatment (group II) had the least regrowth effect as compared to all other experimental groups (Table 2).
In this study, increase in thickness of the subcutaneous layer and the presence of follicles in it were considered as evidence in support of transition of follicles from telogen to anagen hair regrowth phase.
Morphological assessment of the hair regrowth process included estimation of anagen induction. The transition of hair growth from telogen phase to anagen phase was 27.1% in rats treated with Aminexil (group III) and 41.4% in rats treated with Kerium (group II). Anagen induction was the highest in rats treated with minoxidil at 61.7% (group I). In all study groups, the results were compared with the percentage of anagen induction from the untreated side of the animal, which served as control (Table 3). After 4 weeks of topical treatment in group I (minoxidil 2%) an increase in the number of hair follicles (p<0.05 as compared to control area) was noticed in the subcutaneous layer, the majority of which were in the anagen phase and a few in the catagen phase (Table 3, Fig. 4). In group II, (Aminexil) the follicle count in the subcutaneous layer was very low, suggesting that Aminexil induced less hair regrowth. In group II, the anagen induction in the treated areas had similar values as the control areas implying that regrowth was not enhanced by Aminexil treatment (Table 3, Fig. 4). The percentage of anagen induction was significantly increased following topical application of Kerium in group III (p<0.05 as compared to control area) (Table 3, Fig. 4).
On histopathological examination during the anagen phase of hair growth, follicles were located in the deep subcutaneous tissue while in the telogen phase, follicles were present in the dermis itself (Fig. 4).
The results failed to demonstrate significant variation in skin thickness between the control and treated area in any of the four experimental groups (Table 3).
Hair loss and thinning are commonly encountered disorders in clinical dermatology16. Only two Food and Drug Administration (FDA) approved hair loss drugs are available for medical management of hair loss: the dihydrotestosterone- suppressing 5 alfa-reductase inhibitor, finasteride and the antihypertensive potassium channel opener, minoxidil1718. Both finasteride and minoxidil are commonly used in clinical practice. Since finasteride and minoxidil (2% or 5%) have temporary effects and unpredictable efficacy, better pharmacological options for treatment are necessary for managing hair loss19.
There are a number of products claiming to treat hair loss contributing to a multibillion dollar market worldwide. There is an increase in patents for potential useful or doubtful anti-hair loss agents20. Several agents advertised as effective "anti-hair loss" remedies are not supported by convincing studies. This is the reason why "great expectations" turn into plenty of disappointments21. Much of those disappointments appear to result from inefficient drug action and insufficient assessment of the basic pathology of hair loss22. There is also lack of studies regarding the mechanism of action of human hair growth promoters20.
Our study compared several substances that are considered to be hair growth promoters. Since 2% minoxidil is regarded as the gold standard treatment for hair loss it was important to validate minoxidil treatment on a rat model. We also compared the effect of 2% minoxidil, Hairgrow (Dar Al Dawa) with that of Aminexil, Dercos (Vichy Laboratoires), and Kerium, Anti-chute (La Roche-Posay), with focus on trichoscopy, hair weight, and histomorphometric indices (skin thickness, follicle count, anagen ratio). Hair growth cyclicity (anagen, catagen, and telogen phases) can be used both as a diagnosis tool of the hair growth condition, and as a marker of treatment outcome. A unique feature of the hair follicle, cyclic changes involve rapid remodeling of both its epithelial and dermal components1923. The main mesenchymal component, the dermal papilla (DP), plays an important role in inducting new hair follicles and maintaining hair growth24. To pass from the resting phase to the anagen phase, the DP cells demonstrate increased cell division and growth rate. This passage, therefore, requires a good supply of nutrients and a toxin-free environment for the growing cells. If these requirements are not fulfilled, the follicles fail to pass from the resting phase (telogen) to the growing phase (anagen)25.
Taking into consideration the current knowledge about hair cyclicity, we decided to include in our study Aminexil and Kerium topical therapy with the assumption that these compounds promote hair growth. Aminexil, Dercos (Vichy Laboratoires) is the trade name of Kopexil, an N-oxide. Kopexil is a chemical compound similar to minoxidil, but without the piperidine substituent seen in minoxidil. It contains arginine, aminexil, and SP94 peptide. Arginine stimulates microcirculation, bringing in essential nutrients for hair bulb growth. Aminexil (1.5%) helps in reducing the rate of hair loss by not altering the structure of collagen and maintaining the elasticity of the tissue that surrounds the hair root. The SP94 peptide is captured by the hair root and helps in nourishing the hair from its root to the tip by transforming into constructive elements building up the hair fiber. Vitamin B6 is thought to generate beautiful, shining hair that becomes thicker and stronger from within26.
Kerium Anti-chute (La Roche Posay) contains Thermal Spring Water, madecassoside, vitamin B5, and arginine. The cellular nutrients (vitamin B5+arginine) nourish the hair roots and give strength to the fiber, while the La Roche Posay Thermal Spring Water contains anti-free radical Selenium. This enhances the therapeutic action of madecassoside, which in turn inhibits local micro-irritation and its spread to the capillary bulb. Aminexil reduces the accelerated aging of the roots by countering fibrosis and stiffening of collagen sheets and by fastening the hair root within the scalp2122. The treatment needs one application every day for a minimum of 3 applications every week, by using one of the two adapted applicators (for men and women).
The rats used in our experiment were 122~126 days old and therefore, in the telogen phase of hair growth cycle. The experiment lasted four weeks and during this time, the rats passed through an almost complete hair cycle7. According to our results, minoxidil 2% and Kerium treatment contributed to the increase in the number of hair follicles observed in the subcutaneous layer (p<0.05 as compared to the control area), with the majority being in the anagen phase. Similar results were not observed with Aminexil, suggesting that it did not have the same beneficial effects on hair regrowth. Trichoscopy demonstrated that the best hair regrowth was achieved with 2% minoxidil, with treated rats developing type 3 and type 4 patterns of hair regrowth (moderate to high hair density) on test areas.
Previous microscopy studies on mice demonstrated an association between anagen induction and increased skin thickness, follicle count and macroscopic development of skin pigmentation27. In our study, the statistically significant anagen induction (p<0.05) was not accompanied by increase in skin thickness.
Our results confirmed hair regrowth with Kerium as seen on weight assessment and histopathological examination. A similar confirmation of good hair regrowth was not demonstrated in the Aminexil treated group. On trichoscopy, Aminexil and Kerium treated groups had lesser hair growth effects than minoxidil.
An important outcome of our study was the validation of the hair regrowth effect of 2% minoxidil on Wistar rats. Minoxidil, which was first used as an orally administered antihypertensive drug, was associated with interesting side effects of increased hair growth on the scalp and darkening of fine body hairs. In 1988 the FDA approved 2% minoxidil for treating androgenic alopecia in men (for central/vertex hair loss only), and in 1991 its use was permitted for women as well18. Since then, minoxidil has been marketed under many trade names. Minoxidil slows or stops hair loss and promotes hair growth by a mechanism that is still uncertain. Since the telogen phase follicles are shed and are replaced by new anagen hairs, it is possible that minoxidil acts as a nitric oxide agonist. Minoxidil is a potassium channel opener, causing hyperpolarization of cell membranes, thus allowing more oxygen, blood and nutrients to reach the follicle28. Minoxidil is also a vasodilator and increases the cutaneous blood flow to the scalp29. Minoxidil does not decrease dyhydrotestosterone (DHT) or 5-alpha reductase, the enzyme responsible for accumulation of DHT around the hair follicle, ruling out the therapeutic action of minoxidil on the hormonal or genetic causes of hair loss. Minoxidil is administered topically and is available in two concentrations: 2% and 5%30.
The usual dosage is 1 mg per day, applied twice daily to the affected area and followed by slight massage of the scalp. It is advised that the treatment area should not get in contact with water for at least four hours after application in order to achieve maximum results17. Studies suggest ceasing of hair regrowth and onset of hair loss in 30 to 60 days if minoxidil treatment is stopped for more than 6 months30. This happens because without the beneficial environment created by minoxidil, the follicles return to their primary condition, and are exposed to DHT resulting in their shrinkage and destruction. Minoxidil must be used indefinitely for continuous support of existing hair follicles and maintenance of the experienced hair regrowth, if any.
Uno and Kurata31 studied hair growth promoters, minoxidil, diazoxide, and Cooper peptides, on fuzzy rats. Their results with minoxidil demonstrated a conversion of short vellus hairs to prolonged terminal hairs. They also noticed an enlargement of the follicular size with prolongation of anagen phase due to an enhanced rate of cell proliferation.
In our experiment, though Kerium showed a significant increase of anagen induction when compared with control group, this finding was not consistently supported on trichoscopic examination. The explanation could be that trichoscopy is at best an approximation of hair growth and does not provide an exact measurement of newly grown hairs. It cannot quantify minor increases in hair density, (as one cannot count the number of hairs per unit or determine their diameter) which still are considered reliable signs of hair regrowth. Conversely, morphometric evaluation as well as hair weight evaluations are quantitative, objective methods and can determine slight increases in hair density.
Our results suggest that 2% minoxidil topical application is more efficacious than Aminexil or Kerium in inducing hair regrowth as assessed by trichoscopy, hair weight examination, and morphometric assessment. Microscopic data obtained from the validation study confirms that the topical administration of 2% minoxidil affects the normal hair cycle by inducing anagen phase of hair growth in the resting follicles. We used an animal model of hair regrowth to demonstrate that not all the products recommended for human use have the same hair regrowth efficacy. This study validates the hair regrowth effect of minoxidil and also demonstrates that Kerium treatment induces good hair regrowth in Wistar rats.
Figures and Tables
Fig. 4
The effect of the different types of treatments (left column) on hair regrowth in Wistar rats, compared with the negative control area. The dotted lines indicate the junction between dermis (D) and subcutaneous layer (S) (H&E). The columns on the right illustrate the percentage of anagen phase induction for each type of treatment (*p<0.05).
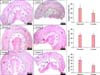
Table 1
Experimental groups and treatments

Table 2
Hair weight assessment mean

| Treatment | Hair weight (mg/cm2) | p-value |
|---|---|---|
| Minoxidil | 44.6±1.1 | 0.018* |
| Control site | 39.7±1.4 | |
| Aminexil | 41.4±1.9 | 0.187 |
| Control site | 37.5±2.2 | |
| Kerium | 48.8±1.7 | 0.012* |
| Control site | 41.6±1.9 |
Table 3
Histopathologic evaluation for morphometry

ACKNOWLEDGMENT
This paper was published under the frame of the European Social Found, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/138776.
References
1. Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001; 19:161–166.

2. Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005; 80:1316–1322.

3. Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004; 123:455–457.

4. Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993; 29:568–575.

5. Poulos GA, Mirmirani P. Investigational medications in the treatment of alopecia. Expert Opin Investig Drugs. 2005; 14:177–184.

7. Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964; 12:465–474.

8. Hynd PI, Schlink AC, Phillips PM, Scobie DR. Mitotic activity in cells of the wool follicle bulb. Aust J Biol Sci. 1986; 39:329–339.

9. Uno H. Quantitative models for the study of hair growth in vivo. Ann N Y Acad Sci. 1991; 642:107–124.
10. Hattori M, Ogawa H. Biochemical analysis of hair growth from the aspects of aging and enzyme activities. J Dermatol. 1983; 10:45–54.

11. Sundberg JP, King LE Jr. Mouse models for the study of human hair loss. Dermatol Clin. 1996; 14:619–632.

12. Ahmad W, Faiyaz ul, Brancolini V, Tsou HC, ul Haque S, Lam H, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998; 279:720–724.

13. Cotsarelis G. Male pattern balding may be due to stem cell inactivation, according to penn study [Home page on the Internet]. Philadelphia: 2011. updated 2011 Jan 4. cited 2012 Sep 12. Available from: http://www.uphs.upenn.edu/news/News_Releases/2011/01/male-pattern-balding-stemcell-inactivation/.
14. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988; 27:8706–8711.

15. Moretti G, Rebora A, Giacometti C, Boido V, Rampini E, Cipriani C. The quantitative behavior of cutaneous histamine and mast cells in the hair cycles of rats. J Invest Dermatol. 1966; 46:231–239.

16. Gordon KA, Tosti A. Alopecia: evaluation and treatment. Clin Cosmet Investig Dermatol. 2011; 4:101–106.

17. Tsuboi R, Tanaka T, Nishikawa T, Ueki R, Yamada H, Katsuoka K, et al. A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur J Dermatol. 2007; 17:37–44.
20. Yoon JI, Al-Reza SM, Kang SC. Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem Toxicol. 2010; 48:1350–1354.

21. Bandaranayake I, Mirmirani P. Hair loss remedies--separating fact from fiction. Cutis. 2004; 73:107–114.
22. Paus R. Therapeutic strategies for treating hair loss. Drug Discov Today Ther Strateg. 2006; 3:101–110.

23. Angelelli L, Cavina G, Moretti G, Siniscalchi P. Quantitative analysis of phospholipids of biochemical and pharmaceutical interest by means of thin layer chromatography: evaluation of the precision of the method. Farmaco Prat. 1966; 21:493–507.
24. Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992; 115:587–593.

25. Courtois M, Loussouarn G, Hourseau C, Grollier JF. Ageing and hair cycles. Br J Dermatol. 1995; 132:86–93.

26. Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008; 59:547–566. quiz 567-568

27. Dry E. The coat of the mouse (Mus musculum). J Genet. 1926; 1:287–340.
28. Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol. 2010; 9:1412–1419.
29. Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AG, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004; 50:541–553.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download