Dear Editor:
Hyaluronic acid (HA) fillers have been widely used for soft-tissue augmentation. Because of HA's biocompatibility and biodegradability, adverse reactions are minimal. However, HA-related complications, such as delayed foreign body granulomas, delayed hypersensitivity, and necrosis have been reported12. We herein present a case of 2 sequential soft facial lumps after injection of a HA filler.
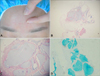
A 33-year-old woman presented with a bean-sized, fleshcolored, soft, movable, subcutaneous mass on her forehead that had been present for 3 months (Fig. 1A). Sixteen months ago, she had been injected with a HA filler (YVOIRE®; LG Life Sciences, Seoul, Korea) on her nose at a private clinic. She had not undergone any filler injection on the forehead. Clinically, the patient was assessed to have an angioma or lipoma and underwent surgical excision of the mass after injection of a local anesthetic agent. Histopathological examination showed the presence of irregular, amorphous, light grayish to bluish material that separated from dispersed collagen bundles with sparse inflammatory cell infiltration in the lower dermis and subcutaneous fat (Fig. 1B, C). The amorphous material stained blue with Alcian blue, pH 2.5 (Fig. 1D). These findings were considered to be consistent with HA. Two weeks after the excision, another, bean-sized, flesh-colored, soft, movable subcutaneous mass was found on the patient's glabella. Surgical removal was also performed, and histologic findings were the same as for the previous biopsy (Fig. 2). At 5 months after treatment by surgical excision, the masses had not recurred.
Foreign body reactions due to HA fillers can occur months or even years after injection. Therefore, a delayed foreign body reaction due to a HA filler is not easy to diagnose. Furthermore, a non-inflammatory, soft, subcutaneous lump distant from the filler injection site would not be considered a HA filler-related problem34. Our patient had 2 sequential, bean-sized, flesh-colored, soft, subcutaneous masses on her face—one on the forehead and one on the glabella—after injection of a HA filler on the nose. She had not undergone filler injection on her forehead and glabella. This phenomenon can be explained by migration of the filler, which refers to the presence of filler at a location remote from the primary injection site. Filler migration can occur by several mechanisms, including poor injection technique (high-volume, high-pressure injection), massage, muscle activity, gravity, antigravity, pressure-induced displacement, lymphatic spread, and intravascular injection4. According to a previous report, low-volume and low-pressure filler injections and more than 1 treatment session are recommended to minimize filler migration5. Additionally, some authors suggest that patients with filler injections limit physical activity and keep the face at rest for the immediate time period after filler injection4.
We report a rare case of 2 sequential facial lumps related to the migration of injected filler. Dermatologists should be aware that dermal fillers, including HA fillers, can migrate to locations distant from the original injection sites.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download