Abstract
Pleomorphic dermal sarcoma (PDS) is a rare mesenchymal neoplasm sharing histopathological features with atypical fibroxanthoma (AFX), but has additional features of deep invasion of the superficial subcutis, tumor necrosis and vascular/perineural invasion. It is not well documented in the literature because of its rarity, and its clinical course has been debated due to the lack of homogenous criteria. We describe here the case of a 91-year-old female with a 6-month history of a solitary, asymptomatic, well-defined, 3.4-cm-sized, reddish, hard, protruding mass on the lateral aspect of the right upper eyelid. On the basis of initial punch biopsy results, storiform cellular infiltrate of pleomorphic spindle and polygonal cells with frequent atypical mitoses, the lesion was identified as AFX. Following the initial biopsy, micrographic surgery was performed and a tumor-free margin was confirmed. Considering the conservation of the periocular function and the advanced age of the patient, we planned secondary intention healing rather than primary suturing. After surgery, skeletal muscle infiltration was found and the diagnosis was revised to PDS by a pathologist based on the currently accepted criteria for PDS. There has been no evidence of recurrence or periocular functional defects during a 2-year follow-up without adjuvant therapy. Although the PDS is highly malignant, complete excision under micrographic surgery can prevent recurrence without adjuvant therapy. Also, the secondary intention healing is an effective method for closure of large defects on the face.
Pleomorphic dermal sarcoma (PDS) and atypical fibroxanthoma (AFX) are rare neoplasms that share histopathological and clinical features. Both tumors exhibit pleomorphic spindle-cell morphology similar to that of other soft-tissue tumors derived from mesenchymal cells, and most tumors arise in the head area1. PDS has the following additional histopathological features: deep invasion of the superficial subcutis, tumor necrosis or vascular/perineural infiltration2. Despite its high-grade morphology, PDS exhibits low-grade malignant behavior1. However, a recent study reported that PDS may be more aggressive than previously estimated3.
Herein, we report a case of PDS with skeletal muscle invasion in the eyebrow area, which was treated by micrographic surgery and secondary intention healing without complications.
A 91-year-old Korean female with a history of hypertension and cerebral infarction presented with an asymptomatic, well-defined, solitary, 3.4-cm-sized, reddish, hard, palpable protruding mass with central crust on the lateral aspect of the right upper eyelid that had been present for 6 months and had increased in size rapidly during the past 3 months (Fig. 1A).
Two punch biopsies at the central and lateral part of the mass were performed. Hematoxylin and eosin staining showed storiform cellular infiltrate of pleomorphic spindle and polygonal cells with frequent atypical mitoses, and so the lesion was suggested to be AFX. Computed tomography performed to evaluate invasion levels showed skin and subcutaneous fat as heterogeneous enhancing mass on the lateral aspect of the right upper eyelid without infiltration of the lymph nodes in the head and neck.
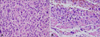
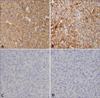
To conserve periocular function and due to the advanced age of the patient, we planned micrographic surgery with secondary intention healing without primary suture rather than a wide excision (>2-cm margin), followed by further adjuvant radiotherapy. During the surgery, complete circumferential peripheral and deep free margins were confirmed by histological examination of frozen sections by a pathologist. Hematoxylin and eosin staining of the excised specimens showed a storiform malignant tumor, which was not connected to the epidermis and composed of tumor cells of variable size and appearance with hyperchromatic nuclei and abundant eosinophilic cytoplasm with frequent bizarre atypical cells and atypical mitoses (Fig. 2A). The peripheral dermis of the tumor showed solar elastosis. Furthermore, focal neoplastic microinfiltrations were seen between bundles of skeletal muscle with moderate inflammatory cell infiltration (Fig. 2B). Immunohistochemical staining demonstrated that the tumor cells were positive for vimentin (Fig. 3A) and CD68 (Fig. 3B); weakly positive for smooth muscle actin (Fig. 3C); and negative for pankeratin (Fig. 3D), HMB-45, desmin, S-100 protein, c-kit, CD31, CD34, and CD99 (Table 1). On the basis of these pathologic findings, the diagnosis was revised to PDS. After four sessions of radiotherapy (total of 8 Gy in four fractions), the patient refused further radiotherapy due to neurological problems because of cerebral disease. The lesion healed completely without complications (Fig. 1B~D), and there has been no evidence of local tumor recurrence during a 2-year follow-up despite no further therapy.
AFX is a dermal-origin neoplasm that presents as a nodular or polypoid tumor on the sun-exposed skin of the elderly, together with ulceration or bleeding. Histopathologically the features are frequently highly atypical cells with marked nuclear pleomorphism and numerous mitotic figures, but AFX usually shows benign clinical behavior with occasional local recurrence45. However, the current World Health Organization classification of soft tissue tumors considers that tumors with morphologic and immunohistochemical features of AFX with invasion beyond the superficial subcutis: the deep subcutis, underlying fascia, or skeletal muscle, lymphovascular or perineural infiltration, and/or tumor necrosis have an increased risk of local recurrence and metastatic disease6. Tumors with adverse features should be regarded as a separate entity from AFX, namely PDS2. This concept has been accepted generally, and the terms "undifferentiated pleomorphic sarcoma (UPS) of skin" or "superficial malignant fibrous histiocytoma (MFH)" have been used. AFX/PDS is distinct from UPS known as so-called MFH in the past, due to its rare metastasis despite histological features malignancy7.
According to the currently accepted criteria for PDS, it presents as a well-circumscribed polypoid, nodular or less frequently, plaque-like mass which may appear pseudoencapsulated and mostly on the head in elderly patients (median age 81 years, range 47~97 years) with rapid growth, and ulceration or bleeding13. Histologically, PDS and AFX are dermal-based lesions with irregular and asymmetric borders and without a grenz zone, epidermal collarette, or epidermal connection. Tumors are cellular and composed of atypical spindle cells admixed with various proportions of pleomorphic epithelioid and scattered multinucleated giant cells, and arranged in major areas of fascicular pattern with minor areas of storiform or haphazard arrangement. The neoplastic cells are large and characterized by plump, palely eosinophilic, and occasionally vacuolated cytoplasm, and often bizarrely shaped moderately to markedly pleomorphic vesicular nuclei with prominent eosinophilic nucleoli. Also, mitotic activity is brisk with frequent atypical figures13.
No cytological or immunohistochemical features enable PDS to be distinguished from AFX, with the exception of the following: extensive infiltration of the subcutis, or invasion into skeletal muscle and underlying fascia or galea; tumor necrosis; and lymphovascular or perineural infiltration8. A study of the genetic correlation between PDS and AFX found that both tumors share telomerase reverse transcriptase promoter mutations, which were found to have an ultraviolet signature (C>T or CC>TT)9. Thus, PDS and AFX are considered to be different forms of the same entity28.
PDS and AFX are diagnosed by excluding other spindle-cell and pleomorphic neoplasms because of their overlapping histopathological features, and no specific immunohistochemical or molecular markers can be used for the differential diagnosis. Nevertheless, immunohistochemistry is indispensable for diagnosis of PDS or AFX, because the absence of cytokeratin, desmin, S100, and CD34 expression is a diagnostic criterion1310. However, other common skin cancers, such as a poorly differentiated squamous cell carcinoma or melanoma, can be distinguished using the appropriate specific markers.
PDS can be distinguished from myofibroblastic and smooth muscle lesions, such as leiomyosarcoma or pleomorphic rhabdomyosarcoma, by the absence of myofibroblastic markers including desmin, calponin, and h-caldesmon1112, and smooth muscle histopathological features13. Myxofibrosarcoma may resemble the areas of myxoid differentiation in PDS, and stain with vimentin and only rarely focally for actin14. However, it is a tumor of subcutaneous tissues with a predilection for the lower extremities of the elderly15. Fibrosarcomatous dermatofibrosarcoma, a variant of dermatofibrosarcoma protuberans, is characterized by a focal fascicular or "herring-bone" pattern, displaying greater cellular atypia and mitotic count. These high-grade sarcoma areas can mimic the storiform pattern of PDS, but show diffuse CD34 expression and lack the marked nuclear pleomorphism evident in dermatofibrosarcoma protuberans.
Complete excision, even with narrow margins, is the treatment of choice for PDS; incomplete excision is a risk factor for local recurrence13. In this case, we performed micrographic surgery, which can enable almost-complete excision in the operation field, and there has been no evidence of local recurrence for 2 years. Also, secondary intensive healing, which is the most elemental form of wound closure, provides several benefits. These include a lower risk of damage and tension to adjacent structures, large defects that require scar extension to remove the standing cones to enable primary closure, and the fact that older patients with more wrinkles and sun damage better accommodate otherwise-more-noticeable scars16.
In conclusion, particularly for tumors suspected to not be AFX or PDS before surgery, micrographic surgery can provide information on the marginal clearance in the operation field, and can clarify the prognosis. After micrographic surgery on several facial structures the function of which must be conserved, such as the eyebrow area, healing by secondary intention is a safe and effective method of closure.
Figures and Tables
Fig. 1
Clinical photograph. (A) Asymptomatic, well-defined, solitary, 3.4-cm-sized, reddish, hard, palpable protruding mass with a central crust on the lateral aspect of the right upper eyelid (before surgery), and (B) 2 weeks, (C) 1 month, and (D) 7 months post-surgery.
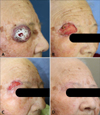
Fig. 2
Pleomorphic dermal sarcoma. (A) High-power view reveals frequent bizarre atypical multinucleated tumor cells and many atypical mitoses (>20/10 HPFs) (H&E, ×400). (B) Individual and aggregated tumor cells invading the skeletal muscle are evident (H&E, ×400).
References
1. Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol. 2012; 36:1317–1326.
2. McCalmont TH. Correction and clarification regarding AFX and pleomorphic dermal sarcoma. J Cutan Pathol. 2012; 39:8.

3. Tardío JC, Pinedo F, Aramburu JA, Suárez-Massa D, Pampín A, Requena L, et al. Pleomorphic dermal sarcoma: a more aggressive neoplasm than previously estimated. J Cutan Pathol. 2016; 43:101–112.

4. Ang GC, Roenigk RK, Otley CC, Kim Phillips P, Weaver AL. More than 2 decades of treating atypical fibroxanthoma at mayo clinic: what have we learned from 91 patients? Dermatol Surg. 2009; 35:765–772.
5. Beer TW, Drury P, Heenan PJ. Atypical fibroxanthoma: a histological and immunohistochemical review of 171 cases. Am J Dermatopathol. 2010; 32:533–540.

6. Calonje JE, Brenn T, Komminoth P. Atypical fibroxanthoma. 4th ed. Lyon: IARC Press;2013.
7. Fletcher CD. The evolving classification of soft tissue tumours-an update based on the new 2013 WHO classification. Histopathology. 2014; 64:2–11.

9. Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, Sucker A, et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol. 2014; 27:502–508.

10. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010; 37:301–309.

11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, Suster S. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006; 28:105–111.

12. Harding-Jackson N, Sangueza M, Mackinnon A, Suster S, Plaza JA. Spindle cell atypical fibroxanthoma: myofibroblastic differentiation represents a diagnostic pitfall in this variant of AFX. Am J Dermatopathol. 2015; 37:509–514. quiz 515-516.
13. Kraft S, Fletcher CD. Atypical intradermal smooth muscle neoplasms: clinicopathologic analysis of 84 cases and a reappraisal of cutaneous "leiomyosarcoma". Am J Surg Pathol. 2011; 35:599–607.
14. Mansoor A, White CR Jr. Myxofibrosarcoma presenting in the skin: clinicopathological features and differential diagnosis with cutaneous myxoid neoplasms. Am J Dermatopathol. 2003; 25:281–286.

15. Merck C, Angervall L, Kindblom LG, Odén A. Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastichistiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand Suppl. 1983; 282:1–40.
16. Zitelli JA. Wound healing by secondary intention. A cosmetic appraisal. J Am Acad Dermatol. 1983; 9:407–415.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download