Abstract
Background
There is evidence that glycosaminoglycans (GAGs) are present in the hair shaft within the follicle but there are no studies regarding GAGs isolation and measurement in the human hair shaft over the scalp surface, it means, in the free hair shaft.
Objective
The purpose of our research was to isolate and measure the total GAGs from human free hair shaft.
Methods
Seventy-five healthy individuals participated in the study, 58 adults, men and women over the age of 50 and 17 children (aged 4~9). GAGs in hair samples, received from the parietal and the occipital areas, were isolated with 4 M guanidine HCl and measured by the uronic acid-carbazole reaction assay.
Hair follicle is a self-renewing organ, composed of epithelial and dermal compartments interacting with each other. The hair matrix keratinocytes produce the epithelial parts of the hair follicle: the hair shaft, the inner root sheath and the outer root sheath (ORS). The dermal part, of mesenchymal origin, consists of dermal papilla and connective tissue sheath, known to be rich in extracellular matrix compounds, such as glycosaminoglycans (GAGs)1234. GAGs are anionic polysaccharide chains, covalently attached to core proteins to form proteoglycans (PGs), except from hyaluronic acid which can be found as a free polymer56.
PGs are present in all mammalian tissues and they represent structural and functional components of the extracellular matrix, of cellular and basement membranes (BMs) and of the skin and its appendages, as the hair follicle78.
Based on previous studies89 dermal papilla is enriched with a BM–chondroitin-sulphate proteoglycan (BM-CSPG), which has a rather stabilizing role; heparin sulphate (HS)-PGs, as perlecan, are present in dermal papilla and follicular BMs; a leucine-rich PG, decorin, is associated with interstitial collagens and may influence fibrillogenesis; the transmembrane PG syndecan is strongly expressed in mesenchymal cells participating in epithelial-stromal interactions during hair follicle morphogenesis. There is evidence that during the hair cycle, versican may interact with secreted growth factors modulating the signalling exchange between the epithelium and the dermal papilla9.
Also, Kloska et al.10 demonstrated changes in hair morphology of patients suffering from mucopolysaccharidosis I (MPS I), indirectly related to dermatansulphate accumulation. The mechanism of abnormal hair fiber formation11 has not been clarified.
Recently, Malgouries et al.12, using immunohistochemistry and immunofluorescence techniques, detected syndecan-1 and CD44v3 in the epithelial part of the hair and in the ORS, while the dermal compartment of the hair follicle was shown to contain large amount of PGs and their GAG chains, such as HS, chondroitin sulphate (CS), dermatansulphate and keratin sulphate. Syndecan-1 and its heparansulphate moiety were expressed in the part of the hair shaft within the follicle; the PG pattern was characterized by discontinuities and disappearance of some epitopes.
Although there are several studies describing PGs and GAGs distribution in the human follicle, to the best of our knowledge, there are no data regarding isolation and quantification of GAGs in the free part of the human hair shaft.
Therefore, we investigated the presence of GAGs in the free hair shaft from healthy volunteers and we examined the possible correlation of GAG levels with participants' age and the scalp region of hair origin.
Seventy-five healthy individuals were selected to participate in the study, 58 adults, men and women over the age of 50 and 17 children (aged 4~9). All participants were clinically free of disease (hair and/or scalp disease included) for the last 6 months at least, as defined by medical history and present physical examination. Routine haematological and biochemical tests were within normal limits. The presence of stable androgenetic alopecia (stages I~III, according to Hamilton classification for males, and stages I~II, according to Ludwig classification for females) was allowed. To limit possible hormonal influence, women were in postmenopausal phase for at least one year before entering the study and children had no clinical signs of puberty. Usual scalp hair manipulation was permitted, but any topical use of herbal or chemical substances, which remain permanently in hair or provoke permanent damages (e.g., hair dyes, permanent, etc.) as well as topical pharmaceutical treatment, were not.
The study was approved by the Ethics Committee of the Medical School of University of Athens (no. 12140/30-07-2008) and all participants (or children's parents) signed a written consent.
Two hair samples were taken from every participant; one from the parietal and another from the occipital area. To assure that we have taken the last 6 months hair, from all participants, and knowing that hair grows at a rate of approximately 1 cm per month13, we left 1 cm from the scalp surface and cut hair of 5 cm length, to reach the total weight of 0.250 g per sample. The samples were stored at room temperature until analysis.
Since there were no previous studies regarding GAGs isolation in hair, we used an extraction protocol with 4 M guanidine HCl, previously described for skin and other tissues1415. Initially, hair was extensively washed with Teen 80 (1%) and distilled water, to remove any debris, and dried at room temperature. Then, hair samples were cut in pieces of 1 cm length approximately and every sample was suspended with 2.5 ml 4 M guanidine HCl/0.05 M sodium acetate (pH 5.8), under continuous stirring for 48 hours at 4℃1516. GAGs were measured in the remaining solution after hair removal and centrifugation at 20,000 g for 20 minutes at 4℃14.
Descriptive statistics are presented as means±standard deviations, medians and ranges, or percentages when appropriate. Because most of the variables were non-normally distributed, we proceeded to the statistical analysis using non-parametric tests. Continuous variables were compared by means of the two-sample Wilcoxon rank-sum (Mann-Whitney) test, the Wilcoxon matched-pairs signed-ranks test, and the Kruskal-Wallis (equality-of-populations) rank test. Linear regression analysis was also performed when the levels of GAGs (per gram of hair shaft) was the outcome of interest. Covariates included in the univariable and the multivariable models were either binomial (gender; alopecia) or continuous variables (age; height; weight; body mass index). For hypothesis testing, a probability level of <0.05 was considered to be statistically significant. All statistical tests were two-sided. Stata software was used for all statistical analyses (Stata Corp., College Station, TX, USA).
The study population included 75 Caucasian healthy volunteers, who were enrolled, interviewed and tested during a 2-year period. The demographic, clinical characteristics, laboratory parameters and GAGs measurements of the study population are summarized in Table 1.
GAGs were isolated in the free hair shaft of all participants, from both scalp regions, parietal and occipital. The levels of GAGs in the hair shaft were not different between the two genders. On the other hand, they were significantly higher in the occipital than in the parietal region of the scalp in the overall study population (n=75; median: 197.0, range: 59.0 to 779.5 vs. median: 120.6, range: 27.6 to 776.6; p<0.001), as well as when the comparison was restricted among the children (n=17; median: 442.3, range: 138.9 to 779.5 vs. median: 139.7, range: 82.8 to 776.6; p=0.002) or among the adults (n=58; median: 157.7, range: 59.0 to 744.8 vs. median: 108.0, range: 27.6 to 412.7; p<0.001).
The GAG levels were not associated with body mass index, hair colour, or type, but were significantly higher especially in the parietal region of the scalp of males with alopecia (n=17; median: 142.6, range: 37.1 to 404.8) as compared to the other healthy adult men (n=15; median: 96.3, range: 27.6 to 211.8; p=0.036).
GAGs in the hair shaft were significantly higher in children (n=17) than in adults (n=58), both in the occipital (median: 442.3, range: 138.9 to 779.5 vs. median: 157.7, range: 59.0 to 744.8; p=0.002) and in the parietal region of the scalp (median: 139.7, range: 82.8 to 776.6 vs. median: 108.0, range: 27.6 to 412.7; p=0.015).
Furthermore, to evaluate the potential explanatory variables independently associated with the levels of GAGs in the hair shaft, we have run univariable and multivariable linear regression analyses. When the analysis was restricted to the adult population, body mass index (beta coefficient: +8.80; p=0.006) were independently associated only with the values of the GAGs in the parietal hair shaft of adults (Table 2, 3). When this analysis was repeated among the children (data not shown), only the body weight was found to be positively associated with the GAG levels in the parietal hair shaft (beta coefficient: +12.6; p=0.026).
We investigated the presence of GAGs in the free hair shaft from healthy volunteers and we examined the possible correlation of GAG levels with participants' age and the scalp region of hair origin. As there was no previous experience of GAGs extraction from hair, a protocol with 4 M guanidine HCl, already performed for skin and other tissues was used1415 (see MATERIALS AND METHODS section). Then, GAGs levels were measured by the UA-carbazole reaction assay. This assay is a modification of an older method of Dische18 based on the reaction of carbazole with the hydrolysed dehydrated derivatives of hexuronic acids. It allows the estimation of glucuronic acid (contained in CS and hyaluronic acid), as well as iduronic acid (contained in GAGs such as dermatan sulphate and heparin). Although it is a high sensitive quantitative method, some limitations should be taken into account: keratan sulphate which does not contain UA, cannot be accounted with this technique14.
We know that hair growth on the scalp occurs in cycles where periods of growth are followed by periods of regression. The androgens19 are the most important regulators of this process for adults with normal thyroid function and sufficient nutrition. They stimulate hair growth in many body areas in men, but they may have an opposite effect on specific hair follicles even in the same person, at different scalp areas. This paradoxical activity of androgens is not fully understood. Therefore, GAGs from parietal and occipital hair shaft samples were measured.
We found that children's free hair shaft contained GAGs. In hair samples collected from the same areas of adults, we have shown GAGs presence, but in lower levels compared to children. It is known that age-related changes occur in human skin GAGs. Also, senescent alopecia20, defined as non androgen-dependent progressive decrease in the number of hair follicles and hair thinning (reduction of hair diameter is observed), found in people of over 50 years of age, as a result of normally cumulative degeneration, is an aging hair phenomenon. It could be suggested that hair GAG decrease in aging hair may be involved in the mechanism of thinning of hair of elderly and that hair GAG alterations follow or at least, are influenced by the overall skin and normal hair aging process15162122.
In both children and adults the occipital hair GAG concentration was higher than the parietal one. Previous studies have proved that there are differences between frontal-parietal (FP) and occipital areas in hormonal receptors' distribution and sensitivity2324. Dermal papilla cells from androgen dependent regions, contain higher levels of androgen receptors (AR) than those from androgen-insensitive areas25. Also, in women with androgenetic alopecia, increased AR expression in the FP region has been found (not observed in normal controls), proving the role of androgen hypersensitivity in this region26. Moreover, a second estrogen receptor, estrogen receptor β (ER β), present in androgen target tissues, as the prostate, has been also, identified in both male and female non balding human scalp skin24. It was supposed that since ERβ may be important in controlling prostate growth, probably by down regulating AR expression27, by extension, it could play a similar role in the human hair follicle and regulate androgen-dependent hair growth, by modulating AR expression or androgen signaling pathways.
According to Randall's hypothesis19, androgens act directly to dermal papilla cells binding to receptors and initiating altered gene expression of regulatory factors within the follicles. Signaling factors such as Wnt and sonic hedgehog28, growth factors, as well as transmembrane and extracellular matrix molecules, seem to be implicated in that process initiated by the androgens29. HS and CS modulate the actions of growth factors such as fibroblast growth factor (FGF)3031, vascular endothelial growthfactor (VEGF)32, bone morphogenetic proteins (BMPs)33, Wnt28 and sonic hedgehog28, which are known to be involved in the biology of the hair follicle1234. It is known that dermal papilla is rich in GAGs but as shown in patients with androgenetic alopecia (AGA)35, androgens activity is related to GAGs reduction. It could be suggested that the occipital privilege of GAGs in our study, may be explained by the non-sensitivity to androgens in this region.
There is evidence that hair growth in anagen in normal scalp is associated with the presence of CS-PGs in the dermal papilla and that regression in catagen is associated to their removal36. Also, versican was almost lost in hair follicles affected by male pattern alopecia, in androgen-dependent areas35. Surprisingly, in our study, parietal hair from men with androgenetic alopecia had higher GAG levels when compared to men without alopecia, but still lower than in the occipital area. Further studies should confirm these data and explore possible alterations in the environment of the remaining follicles in people with androgenetic alopecia that could lead to increased GAG levels in this area.
No significant difference in GAG levels was detected between the two genders comparing hair samples from the same areas. We know that estrogens influence hair growth: they can prolong the anagen phase, e.g., in pregnancy, while female pattern alopecia is most frequently observed after menopause when estradiol production is minimal37. As our females were in menopause, the influence of estrogens on their hair was assumed to be limited. This parameter may explain why the levels of hair GAGs were not different between men and women in our study. Hair shaft GAG levels were not altered by different natural color (black, blond, brown, other) or type (straight, curly, wavy). GAGs from parietal hair were correlated with BMI in adults and body weight in children. As FP area is the region of androgenetic alopecia expression, this finding suggests that metabolic factors could be involved in the pathogenesis and treatment of androgenetic alopecia. Several previous studies have associated androgenetic alopecia with metabolic syndrome38 or insulin resistance presence39. Also, low zinc serum levels40 have been found to be related with hair loss. It could be suggested that the parietal area may be sensitive to metabolic disturbances. Additional studies should explore the role of GAGs in the whole process.
In conclusion, this descriptive study should be considered initial, as it is the first to report the presence of GAGs in the free hair shaft, as well as a preliminary quantitative estimation of hair GAG levels.
Although GAGs are necessary for hair keratins production4142, specific GAG function in hair shaft has to be elucidated. Studies of the hair cycle revealed reduction of GAGs in the connective tissue around the follicle, in patients with hair loss. This reduction was associated with abnormal fragility of scalp hair, suggesting a process of keratin degradation4243. We can hypothesize that GAGs in hair shaft are important for structure and density maintenance.
We believe that the reported presence of GAGs in the hair shaft is of importance and may have special implication in the constant research for the development of new formulas for effective hair care products. Problems of aging hair21 as thinning, dryness and weathering are very frequent. Dry hair does not have enough moisture and loses its shine. This happens because of cuticle damage after chemical and cosmetic procedures such as coloring, permanent, straightening etc, or excessive brushing when hair is wet. As cuticle becomes porous, the hair cortex cannot retain humidity. Different amino acids profile has been identified in normal and weathered hair21. GAGs have the ability to bind to water and peptides. This characteristic indicates that they could be target or vehicle molecules and potential anti-aging agents for hair: lost amino acids and humefactant agents could be delivered back to the hair shaft from cosmetic preparations, as shampoos and conditioners44.
Moreover, our data has shown the correlation of hair GAG levels with participants' age. We believe that age-related hair GAGs changes may affect hair penetration and absorption of herbal or chemical substances and topical therapeutic agents. Thus, new hair care products and treatments should be tailored to age.
Finally, our findings may be proved clinically significant, at least for MPS studying. MPSs are inherited disorders caused by specific enzymatic defects involved in GAGs degradation. In MPS I, II, and III types hair abnormalities have been described11. Recently, hair morphology changes of MPS I patients disappeared after enzyme replacement therapy with recombinant human α-L-iduronidase, the improvement being correlated with urinary GAGs normalization. A hair abnormality score, based on scanning electron microscopy analysis, was proposed as a potential parameter for monitoring the efficacy of MPS treatment10. Although hair abnormalities are not necessary correlated with disease clinical severity, we believe that our quantitative determination of hair GAG levels could be a useful, non-invasive, inexpensive tool for assessing existing or future therapies for MPS. In such a case, it would be interesting to further explore whether it could be also, utilized as a marker of determining the most efficacious dose for new MPS drugs.
Overall, future studies should explore the role of GAGs, if any, in hair as: (a) factors of importance for hair homeostasis, (b) informative parameters for hair aging, (c) potential hair anti-aging agents, (d) target or vehicle molecules for future therapies and cosmetic preparations, (e) clinical predictors of hair treatments' responsiveness.
Figures and Tables
Table 1
Characteristics of the study population

Table 2
Evaluation of potential explanatory variables for levels of GAGs among adults (linear regression analysis' results)
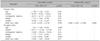
Table 3
GAGs in hair shaft according to scalp regions

ACKNOWLEDGMENT
This work was supported by the Special Account for Research Grants of National and Kapodistrian University of Athens (grant number 70/03/4806).
References
1. Bernard BA. Hair cycle dynamics: the case of the human hair follicle. J Soc Biol. 2003; 197:57–61.
2. Couchman JR. Rat hair follicle dermal papillae have an extracellular matrix containing basement membrane components. J Invest Dermatol. 1986; 87:762–767.

3. Chiu HC, Chang CH, Chen JS, Jee SH. Human hair follicle dermal papilla cell, dermal sheath cell and interstitial dermal fibroblast characteristics. J Formos Med Assoc. 1996; 95:667–674.
4. Braun Falco O. The histochemistry of the hair follicle. In : Montagna W, Ellis RA, editors. The biology of hair growth. Providence, RI: Academic Press;1958. p. 65–90.
5. Schmid K, Grundboeck-Jusco J, Kimura A, Tschopp FA, Zollinger R, Binette JP, et al. The distribution of the glycosaminoglycans in the anatomic components of the lung and the changes in concentration of these macromolecules during development and aging. Biochim Biophys Acta. 1982; 716:178–187.

6. Gniadecka M, Faurskov Nielsen O, Christensen DH, Wulf HC. Structure of water, proteins, and lipids in intact human skin, hair, and nail. J Invest Dermatol. 1998; 110:393–398.

7. Paulsson M. Basement membrane proteins: structure, assembly and cellular interactions. Crit Rev Biochem Mol Biol. 1992; 27:93–127.

9. du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol. 1995; 105:426–431.

10. Kloska A, Bohdanowicz J, Konopa G, Tylki-Szymńska A, Jakóbkiewicz-Banecka J, Czartoryska B, et al. Changes in hair morphology of mucopolysaccharidosis I patients treated with recombinant human alpha-L-iduronidase (laronidase, Aldurazyme). Am J Med Genet A. 2005; 139:199–203.
11. Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000; 156:925–938.
12. Malgouries S, Thibaut S, Bernard BA. Proteoglycan expression patterns in human hair follicle. Br J Dermatol. 2008; 158:234–242.

13. Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009; 34:32–37.

14. Carney LS. Proteoglycans. In : Chaplin MF, Kennedy JF, editors. Carbohydrate analysis: a practical approach. Oxford: Oxford University Press;1987. p. 97–142.
15. Carrino DA, Sorrell JM, Caplan AI. Age-related changes in the proteoglycans of human skin. Arch Biochem Biophys. 2000; 373:91–101.

16. Jung JW, Cha SH, Lee SC, Chun IK, Kim YP. Age-related changes of water content in the rat skin. J Dermatol Sci. 1997; 14:12–19.

20. Kligman AM. The comparative histopathology of male-pattern baldness and senescent baldness. Clin Dermatol. 1988; 6:108–118.

22. Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006; 12:145–154.

23. Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. J Endocrinol. 1992; 133:141–147.

24. Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O'Driscoll J, et al. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc. 2003; 8:100–103.

25. Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998; 156:59–65.

26. Richeti F, Kochi C, Rocha MN, Sant'Anna Corrêa C, Lazzarini R, Guazzelli RM, et al. Increased androgen receptor messenger RNA in frontal-parietal hair follicles of women with androgenetic alopecia. Genet Mol Res. 2013; 12:1834–1840.

28. Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004; 131:1927–1938.

29. Mou C, Jackson B, Schneider P, Overbeek PA, Headon DJ. Generation of the primary hair follicle pattern. Proc Natl Acad Sci USA. 2006; 103:9075–9080.

30. Pellegrini L. Role of heparan sulfate in fibroblast growth factor signalling: a structural view. Curr Opin Struct Biol. 2001; 11:629–634.

31. Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 2002; 290:169–176.

32. Cohen T, Gitay-Goren H, Sharon R, Shibuya M, Halaban R, Levi BZ, et al. VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin binding ability, requires cell-surface heparan sulfates for efficient binding to the VEGF receptors of human melanoma cells. J Biol Chem. 1995; 270:11322–11326.

33. Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003; 278:43229–43235.

34. Catlow K, Deakin JA, Delehedde M, Fernig DG, Gallagher JT, Pavão MS, et al. Hepatocyte growth factor/scatter factor and its interaction with heparan sulphate and dermatan sulphate. Biochem Soc Trans. 2003; 31:352–353.

35. Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005; 39:147–154.

36. Westgate GE, Messenger AG, Watson LP, Gibson WT. Distribution of proteoglycans during the hair growth cycle in human skin. J Invest Dermatol. 1991; 96:191–195.

37. Verdier-Sévrain S. Effect of estrogens on skin aging and the potential role of selective estrogen receptor modulators. Climacteric. 2007; 10:289–297.

38. Su LH, Chen TH. Association of androgenetic alopecia with metabolic syndrome in men: a community-based survey. Br J Dermatol. 2010; 163:371–377.

39. González-González JG, Mancillas-Adams LG, Fernández-Reyes M, Gómez-Flores M, Lavalle-González FJ, Ocampo-Candiani J, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol (Oxf). 2009; 71:494–499.

40. Skalnaya MG, Tkachev VP. Trace elements content and hormonal profiles in women with androgenetic alopecia. J Trace Elem Med Biol. 2011; 25:Suppl 1. S50–S53.

41. Sylven B. The qualitative distribution of metachromatic polysaccharide material during hair growth. Exp Cell Res. 1950; 1:582–589.

42. Moretti G, Cipriani C, Rebora A, Rampini E, Crovato F. Correlation of tissue mucopolysaccharides with the hair cycle. J Invest Dermatol. 1967; 48:498–503.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download