Dear Editor:
Hyaluronic acid (HA) is a high-molecular-weight polysaccharide found throughout the extracellular matrix and is one of the major components of articular joints. Intra-articular injection of HA is often performed for the treatment of symptomatic osteoarthritis. The efficacy of HA injection in relieving pain and improving function in patients with osteoarthritis has been reported previously. Adverse reactions are usually limited to local reactions at the inoculation site, including transient local tenderness1. Although HA has been acknowledged as safe, there have been many reports related to a variety of adverse effects to injectable HA234.
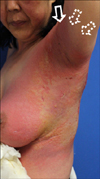
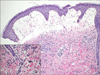
A 59-year-old female presented with diffuse skin eruption with pruritus on the left arm and trunk. She had osteoarthritis and intra-articular injection of HA was performed on her left shoulder in order to relieve left shoulder pain 5 days prior. This was the first time for the patient to be injected by HA. There was no relevant contact history and no history of dermatologic disease. Physical examination revealed diffuse edematous erythema, vesicles and bullae with localized erosion on her left arm and trunk (Fig. 1). Blood sampling showed mild leukocytosis (9,890/µl), but all other results were within normal limits. Histopathologically, severe papillary dermal edema and superficial perivenular mixed inflammatory cell infiltrates were observed with an increased number of eosinophils (Fig. 2). The patient was diagnosed with an acute allergic reaction accompanying cellulitis. Within 1 week of short-term systemic corticosteroid and antibiotic therapy, the cutaneous lesions resolved.
Intra-articular HA injection is a universal, safe non-surgical therapy indicated for patients with osteoarthritis. Adverse events following intra-articular injection of HA are unusual. Most reactions are transient, local erythematous responses including pain, tenderness, edema and heat sensation1.
There have been some previous reports related to the dermatologic side effects of injectable HA. A 70-year-old female patient manifested a generalized target-like rash with confluent edema after intra-articular HA injection and exhibited a positive reaction on an intradermal test for HA2.
She was diagnosed with erythema multiforme due to HA. Another patient, a 68-year-old woman, had a history of knee osteoarthritis and demonstrated knee swelling, painful erythema and bullae with desquamation after intra-articular HA injection4. Histologic findings showed subcorneal cleavage, she was positive for exfoliative toxin B and bacterial culture grew methicillin-sensitive Staphylococcus aureus, supporting a diagnosis of staphylococcal scaled skin syndrome. There was also a report describing a delayed granulomatous response after injection of HA used as a soft tissue filler3. This patient developed a subepidermal blister with histopathologic features that varied from those above.
HA has no known immunogenic side effects, but it is possible to develop anti-HA antibodies5. An allergic reaction can develop resulting in clinically severe erythema, blisters and histopathologic subepidermal cleavage with perivascular lymphocytes and eosinophils in the patient. Unfortunately, the assay for antibodies against HA was not performed in this case. In addition, we did not perform the patch test because of the patient's refusal and worry about systemic adverse reaction. The lack of these tests could not fully explain the allergic reaction. There is also a possibility of allergic reaction for antiseptic agent such as potadine. The pathomechanism is still unknown although the foreign body or hypersensitivity reaction may be hypothesized. Further elucidation is needed.
We introduce a case of severe cutaneous adverse reaction triggered by HA intra-articular injection; serious side effects of this procedure are rare with only a few reported cases. This report indicates that a relatively common therapeutic procedure with low immunogenicity can trigger severe systemic allergic reactions.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download