Abstract
CD30+ lymphoproliferative disorders (LPD) represent a spectrum of T-cell lymphoma including lymphomatoid papulosis and anaplastic large cell lymphoma (ALCL). Epidermis overlying cutaneous CD30+ LPD often shows epidermal hyperplasia, hyperkeratosis, crusting, and ulceration and it is difficult to distinguish from carcinoma such as keratoacanthoma (KA) or squamous cell carcinoma (SCC). Several cases of pseudocarcinomatous hyperplasia mimicking KA or SCC in CD30+ LPD have been reported. The relationship between CD30+ LPD and epithelial proliferations has not yet well understood. It was reported that a variety of mediators, including epidermal growth factor (EGF), transforming growth factor-α and EGFR from CD30+ LPD could attribute to epidermal hyperplasia. However, separate and distinct SCC occurring in CD30+ LPD has rarely been reported. Herein, we present a rare case of coexistence of SCC and cutaneous ALCL located on the same region.
Cutaneous CD30+ lymphoproliferative disorders (LPD) denotes a group of primary cutaneous T-cell lymphomas characterized by expression of the CD30 antigen immune-phenotypically and a favorable prognosis clinically, including lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (ALCL). It has been known that pseudocarcinomatous hyperplasia (pseudoepitheliomatous hyperplasia) can be seen in CD30+ LPD12. Pseudocarcinomatous hyperplasia histologically mimics squamous cell carcinoma (SCC) and the diagnosis of this entity depends on awareness of the condition and a careful survey of a predisposing lesion. However, distinct SCC has rarely been reported in CD30+ ALCL13.
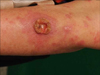
A 60-year-old woman presented with a several weeks history of a crater like tumor on the left forearm. She had a past medical history of primary cutaneous ALCL presenting with masses on the chest and back 5 years ago, which were excised totally. On examination, she had a 15 mm diameter keratoacanthoma (KA)-like tumor with central ulceration on the left forearm, with a background of erythematous papules and plaques (Fig. 1). An incisional biopsy was performed. Laboratory tests including complete blood count, urinalysis, and chemistry profile were unremarkable.
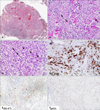
Pathologic finding revealed an exo-endophytic epidermal hyperplasia with marked hyperkeratosis and dense cellular infiltrate in the dermis (Fig. 2A). Epidermal hyperplasia was composed of keratinocytes with abundant pale cytoplasm, microabscesses and horn cysts. The border of epidermal proliferation was bulbous and irregular. In high power view, keratinocytes showed pleomorphism and nuclear atypia and there were conspicuous keratinization and invasive cells dissecting collagens (Fig. 2B). Dermis showed a dense mixed cellular infiltrate. Atypical lymphoid cells were obscured by a massive infiltrate of neutrophils and eosinophils (Fig. 2C). The large atypical cells had pleomorphic, hyperchromatic nuclei and basophilic cytoplasm and were positive for CD30 (Fig. 2D) and CD4. The expression of Ki-67 was scattered in epidermis and large cells of dermis (Fig. 2E). The expression of p53 was diffuse and scattered throughout epidermis (Fig. 2F). The histopathology confirmed a separate and distinct SCC. The SCC was excised widely and reconstructed with spilt thickness skin graft from left anterior thigh. The CD30+ ALCL was treated by radiation. After operation, positron emission tomography-computed tomography showed only mild hypermetabolic uptake in the left proximal forearm. This is a rare case of synchronous occurrence of CD30+ ALCL and SCC.
Cutaneous CD30+ LPD is a group of primary cutaneous T-cell lymphomas. In a small number of CD 30+ LPD, the overlying epidermis shows epidermal hyperplasia. Pseudocarcinomatous hyperplasia overlying cutaneous CD30+ LPD shows hyperkeratosis, irregular acanthosis, crusting, and ulceration3. Therefore, distinction from the SCC can be very difficult histopathologically. Several cases of pseudocarcinomatous hyperplasia overlying CD30+ LPD mimicking KA or SCC have been reported but definite epidermal malignancy in CD30+ LPD occurred rarely1234. It is known that p53 and p63 express in both basal and suprabasal area of SCC and Ki-67 was diffuse and scattered throughout the thickness of the epidermis in a SCC4. However, data of SCC in CD30+ LPD are not sufficient. Therefore, it is possible to mistake pseudocarcinomatous hyperplasia for SCC or KA and vice versa4.
The relationship between CD30+ LPD and epithelial proliferations has not yet well understood. It was reported that a variety of mediators, including epidermal growth factor (EGF), transforming growth factor-α, and EGF- receptor (EGFR) could attribute to epidermal hyperplasia overlying CD30+ LPD5. Cespedes et al.1 explained that CD30+ lymphoid cells might indirectly inhibit an apoptotic homeostatic mechanism expressed by keratinocytes through the cytokines, directly induce proliferation of keratinocytes in a fashion similar to the CD30+ lymphoid cells proliferation, and produce EGF-like molecules to induce epithelial proliferation. Cowley et al.6 proved that EGFR was up-regulated in malignant squamous cell lines. Various mechanisms of the aforementioned are presumed to play a role in occurrence of epidermal proliferation in CD30+ LPD and can rarely cause definite epidermal malignancy including KA or SCC. But, on the other hand, Fernandez-Flores78 purposed that CD30+ cells were common component of the inflammatory infiltrate of KA and SCC and might be a role in KA regression. This was a limited opinion purposed by few physicians78. In this case, her past medical history and pathologic results were favored primary cutaneous ALCL. Based on clinical manifestation suggesting KA and pathologic finding of definite atypical keratinocytes dissecting collagen bundle, it was diagnosed with SCC, ruling out pseudocarcinomatous hyperplasia.
This case implied that epidermal malignancy as well as pseudocarcinomatous hyperplasia in CD30+ LPD can occur simultaneously and meticulous microscopic evaluation might be essential for differential diagnosis.
Figures and Tables
Fig. 2
(A) Histological analysis of the shave biopsy specimen shows exo-endophytic epidermal proliferation with hyperkeratosis, horn cysts, and microabscesses and dense cellular infiltrates in dermis (H&E, ×40). (B) Invasive atypical cells dissecting collagen bundle suggested squamous cell carcinoma (arrows; H&E, ×200). (C) Large atypical cells were obscured by a massive infiltrate of neutrophils and eosinophils in dermis (arrows; H&E, ×200). (D) Large atypical cells are positive for CD30 (CD30, ×200). (E) Expression of Ki-67 was diffuse in epidermis and large cells of dermis. (F) The expression of p53 was diffuse and scattered throughout epidermis.
References
1. Cespedes YP, Rockley PF, Flores F, Ruiz P, Kaiser MR, Elgart GW. Is there a special relationship between CD30-positive lymphoproliferative disorders and epidermal proliferation? J Cutan Pathol. 2000; 27:271–275.

2. Kawachi Y, Taguchi S, Fujisawa Y, Furuta J, Nakamura Y, Ishii Y, et al. Epidermal pseudocarcinomatous hyperplasia with underlying epidermal growth factor-producing cutaneous CD30-positive lymphoproliferative disorder. J Eur Acad Dermatol Venereol. 2009; 23:181–183.

3. Yanagi T, Shimizu T, Kodama K, Nemoto-Hasebe I, Kasai M, Shimizu H. CD30-positive primary cutaneous anaplastic large-cell lymphoma and definite squamous cell carcinoma. Clin Exp Dermatol. 2009; 34:e293–e294.

4. Price A, Miller JH, Junkins-Hopkins JM. Pseudocarcinomatous hyperplasia in anaplastic large cell lymphoma, a mimicker of poorly differentiated squamous cell carcinoma: report of a case and review of the literature. J Cutan Pathol. 2015; 42:863–869.

5. Courville P, Wechsler J, Thomine E, Vergier B, Fonck Y, Souteyrand P, et al. Pseudoepitheliomatous hyperplasia in cutaneous T-cell lymphoma. A clinical, histopathological and immunohistochemical study with particular interest in epithelial growth factor expression. The French Study Group on Cutaneous Lymphoma. Br J Dermatol. 1999; 140:421–426.

6. Cowley GP, Smith JA, Gusterson BA. Increased EGF receptors on human squamous carcinoma cell lines. Br J Cancer. 1986; 53:223–229.

7. Fernandez-Flores A. CD30+ cell population in common keratoacanthomas: a study of 21 cases. Rom J Morphol Embryol. 2008; 49:159–162.
8. Fernandez-Flores A. CD30+ cells in regressing keratoacanthoma and in non-keratoacanthomatous squamous cell carcinoma. Bratisl Lek Listy. 2008; 109:508–512.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download