Abstract
Primary penile melanomas are rare tumors that represent less than 0.1% of all melanomas. We report a case of a 60-year-old Japanese male with a mucosal penile melanoma and describe an increased CD8+ T cell infiltration in brain after dacarbazine (DTIC) administration. After partial penectomy and left inguinal lymphadenectomy, he developed multiple lung, bone, spleen, brain and skin metastases. He was treated with interferon-β, DTIC and nivolumab. However, the metastases were not reduced in size. Immunohistochemistry showed an increase of CD8+ T cell infiltration and programmed death-ligand 1 (PD-L1) expression after the administration of DTIC, but the expression of programmed cell death protein 1 (PD-1) was negative. We speculate that DTIC exerted immunostimulatory effects, but nivolumab was ineffective due to the negative expression of PD-1 and/or an insufficient infiltration of CD8+ T cells. Although this is only one case, this case report could be the first step to discuss the development of effective therapies against melanoma to take advantage of the increased CD8+ T cell infiltration elicited by chemotherapeutic agents. It would be beneficial to pay more attention to the relationship between DTIC and immune checkpoint modulators.
Primary penile melanomas are rare tumors that represent less than 0.1% of all melanomas and their prognosis is quite poor1. The development of effective therapeutic modalities against penile melanomas is imperative. Here, we present a 60-year-old Japanese male with a mucosal penile melanoma treated with dacarbazine (DTIC) and nivolumab. Previous studies show that DTIC exerts immunostimulatory effects by inducing the local activation of natural killer (NK) and T cells2. We found an increased CD8+ T cell infiltration after the administration of DTIC. Although this is only one case, this case report could be the first step to discuss the development of effective therapies against melanomas to take advantage of the increased CD8+ T cell infiltration elicited by chemotherapeutic agents. Our results emphasize the importance of the classification of patients and the discovery of appropriate diagnostic markers for that classification.
A 60-year-old Japanese male presented with a pigmented nodule at his urethral orifice and palpable nodes at his left inguinal lymph node (Fig. 1A). The patient had a history of urination trouble and hematuria a half year before the pigmentation developed. A diagnosis of penile melanoma had been made from a biopsy at the previous hospital and he was referred to Department of Dermatology, Tokyo Medical and Dental University in May 2014. A partial penectomy and left inguinal lymphadenectomy had been performed. Histopathological findings showed melanin deposition and an atypical melanocytic proliferation arranged in sheets and in nests (Fig. 1B). The tumor cells had an eosinophilic cytoplasm and vesicular pleomorphic nuclei with prominent nucleoli and frequent mitoses (Fig. 1C). Immunohistochemical examinations showed positive reactions for Melan-A, HMB-45 and S-100. A histopathological diagnosis of penile melanoma was made. Immunostaining for CD8 revealed that few CD8+ T cells had infiltrated into the primary tumor (4.70±4.75 cells/field) and few CD8+ T cells were observed at the periphery of the tumor (16.15±12.16 cells/field), using a definition of "peripheral" as "both edge and its surrounding of the tumor" (Fig. 2A). Immunostaining for programmed cell death protein 1 (PD-1) revealed a negative reaction on lymphocytes (Fig. 2B). Immunostaining for programmed death-ligand 1 (PD-L1) revealed a slight positive reaction on tumor cells (Fig. 2C). In October 2014, multiple lung metastases and splenic metastases were suspected by contrast-enhanced computed tomography (CT) (Fig. 3A), and positron emission tomography (PET)-CT revealed multiple lung, bone and splenic metastases. From November 2014, one course of DTIC 1,000 mg/m2 and 4 courses of interferon (IFN)-β 3.0×106 IU local injection had been started. However, a metastatic lesion at the left frontal lobe of his brain was detected by magnetic resonance imaging. Tumorectomy in addition to γ-knife therapy was then performed. Immunostaining revealed an increased peripheral infiltration of CD8+ T cells in the brain metastatic lesion (44.65±24.53 cells/field). This infiltration was significantly increased compared to the primary tumor (p<0.0001) (Fig. 2D, J, K). Immunostaining for PD-1 revealed a negative reaction (Fig. 2E) and immunostaining for PD-L1 revealed a slight positive reaction (Fig. 2F). According to PET-CT and contrast-enhanced CT in December, a local relapse on the remaining penis was found, and multiple lung and bone metastatic lesions and a splenic metastatic lesion had become larger than the last time (Fig. 3B). From January 2015, a first course of nivolumab administration was started. However contrast-enhanced CT after that treatment showed an increased numbers of lung metastatic lesions, a metastasis to the left Gerota fascia and a slight enlargement of the splenic metastatic lesion (Fig. 3C). From February 2015, a second course of nivolumab administration was started. However, we discovered several subcutaneous nodules, which were considered to be new metastases, on his nasolabial sulcus and the right medial surface of his thigh. We performed a biopsy from a nodule (6 mm) at the nasolabial sulcus. Immunostaining revealed a CD8+ T cell infiltration at the peripheral site (18.20±10.16 cells/field) and inner site (6.15±6.54 cells/field) of the tumor. The increase of CD8+ T cell infiltration in the skin metastasis was not significant compared to the primary tumor (Fig. 2G, J, K). Immunostaining for PD-1 revealed a negative reaction (Fig. 2H). Immunostaining for PD-L1 revealed a slight positive reaction (Fig. 2I). We evaluated the effect for a second course of nivolumab administration by CT. The number of lung metastases had increased dramatically and a splenic metastatic lesion had become 33 mm (Fig. 3D). We estimated that the response to nivolumab administration was progressive disease (PD).
The continuation of a treatment has come to be difficult due to a rapid enlargement and an increased number of metastases in the brain. He was discharged in order to transfer to the previous hospital for the purpose of palliative therapy.
Chemotherapeutic agents such as DTIC are capable of inducing tumor cell death and shrinking tumor masses while concurrently facilitating antigen uptake and subsequently activating tumor-resident dendritic cells that prime antigen-specific CD4+ and CD8+ T cells3. Previous studies suggest that DTIC exerts immunostimulatory effects by inducing the local activation of NK and T cells2. DTIC triggers the up-regulation of NKG2D ligands on melanoma cells, leading to NK cell activation and IFN-γ secretion by engagement of NKG2DL and NKG2D on NK cells. The NK cell-derived IFN-γ subsequently favors the up-regulation of major histocompatibility complex class 1 molecules on tumor cells, rendering them sensitive to cytotoxic CD8+ T cells24. In our case, we found an increased peripheral CD8+ T cell infiltration in a brain metastatic lesion after the administration of DTIC (Fig. 2D). Compared with the primary tumor, the infiltration of CD8+ T cells was significantly increased (p<0.0001). This observation may suggest a potentiation of the cancer immune response by a chemotherapeutic agent. One study showed that tumor-associated PD-L1 confers resistance to CD8+ cytotoxic T lymphocyte lysis5. This resistance system could be eliminated by blockade of PD-L1 and/or PD-1; however, other molecules such as PD-L2 also participate in this resistance mechanism. As for PD-1 expression, none of these 3 lesions was PD-1 positive in this patient (Fig. 2B, E, H). We speculate that there are two major reasons why nivolumab was ineffective. One reason is that, since PD-1 was negative, other molecules on CD8+ T cells that participate in immune checkpoint regulation such as LAG-3 or TIM-3 may be key molecules in this patient and the blockade by nivolumab was ineffective. The other reason is the insufficient induction of CD8+ infiltration in order to induce sufficient responses to suppress tumor growth. We also speculate that the decrease of CD8+ T cell infiltration from the brain metastasis to the skin metastasis might be due, at least in part, because the immunostimulatory effect of DTIC couldn't last since the DTIC administration was 5 months before the skin metastasis.
Although we cannot deny that the increased infiltration of CD8+ T cells might be due to clonal heterogeneity of melanomas and statistical significances were not exactly rigorous due to a comparison from different metastatic sites including skin and brain, this case is still of great interest because there's few reports67, regarding the increased CD8+ T cells infiltration after an administration of DTIC and a limitation of obtaining biopsy specimens from appropriate metastatic sites of melanoma patients. Recent studies have revealed that immune checkpoint modulators have better effects in cases of high intratumoral CD8+ T cell infiltration8, so the classification of melanoma patients in terms of the effectiveness of nivolumab and its markers to distinguish nivolumab-sensitive patients will be imperative. We also should be cautious about an increased CD8+ T-cell infiltration by nivolumab in analyzing data in order to exclude unnecessary biases. If a strategy targeting PD-1 is ineffective due to the low expression of PD-1 in a melanoma patient, alternative strategies targeting other molecules participating in immune checkpoints should be considered. Although this is only one case, this could be the first step for us to speculate that a novel strategy to control metastatic sites of melanomas in nivolumab-insensitive patients is to take advantage of the enhancing effect on CD8+ T cell infiltration by DTIC. The administration of DTIC prior to nivolumab should have a benefit in a subset of melanoma patients. An appropriate selection of melanoma patients in addition to the development of appropriate markers to identify those patients is important.
In conclusion, we presented a case with an increased CD8+ T cell infiltration after the administration of DTIC. We speculate that this supports a potentiation of the cancer immune response by chemotherapeutic agents. This analysis based on different metastatic sites including skin and brain, and a bias due to clonal heterogeneity of melanomas is not exactly excluded in this study. An accumulation of such cases is required to substantiate this result by correcting those biases and eventually develop more effective strategies as therapeutics using immune checkpoint modulators such as nivolumab.
Figures and Tables
Fig. 1
(A) A pigmented nodule on the urethral orifice (indicated by the white arrow). (B) An atypical melanocytic proliferation arranged in sheets and in nests. (H&E, ×40). (C) Eosinophilic cytoplasm and vesicular pleomorphic nuclei with prominent nucleoli and frequent mitoses (H&E, ×200).

Fig. 2
Immunohistochemistry for CD8 in the primary tumor (A), the brain metastatic lesion (D) and a skin metastatic lesion (G). Immunohistochemistry for programmed cell death protein 1 in the primary tumor (B), the brain metastatic lesion (E) and a skin metastatic lesion (H). Immunohistochemistry for programmed death-ligand 1 in the primary tumor (C), the brain metastatic lesion (F) and a skin metastatic lesion (I) (A~I: ×100). Number of cells staining for CD8 expressed as means±standard deviations measured over 10 high-power fields (×400) in the peripheral (J) and inner (K) layer of the tumor. Counting was performed independently by two observers. *p<0.0001.
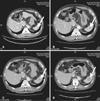
Fig. 3
Contrast-enhanced computed tomography for a splenic metastatic lesion (A) before dacarbazine administration, after dacarbazine administration (B), after the first course of nivolumab administration (C), and after the second course of nivolumab administration (D). Diameters of the splenic metastatic lesion (indicated by white arrows) are 13 mm (A), 20 mm (B), 21 mm (C) and 32 mm (D), respectively. Arrows indicate splenic metastatic lesions.
References
1. Oliva E, Quinn TR, Amin MB, Eble JN, Epstein JI, Srigley JR, et al. Primary malignant melanoma of the urethra: a clinicopathologic analysis of 15 cases. Am J Surg Pathol. 2000; 24:785–796.
2. Ugurel S, Paschen A, Becker JC. Dacarbazine in melanoma: from a chemotherapeutic drug to an immunomodulating agent. J Invest Dermatol. 2013; 133:289–292.

3. Müller P, Martin K, Theurich S, von Bergwelt-Baildon M, Zippelius A. Cancer chemotherapy agents target intratumoral dendritic cells to potentiate antitumor immunity. Oncoimmunology. 2014; 3:e954460.

4. Hervieu A, Rébé C, Végran F, Chalmin F, Bruchard M, Vabres P, et al. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol. 2013; 133:499–508.

5. Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005; 65:1089–1096.
6. Ozawa A, Nomiyama T, Nakai N, Hartmann G, Takenaka H, Kishimoto S, et al. Immunohistological analysis of in-transit metastasis in a patient with advanced melanoma treated with combination therapy of cytosine guanine dinucleotide oligodeoxynucleotide, dacarbazine and beta-interferon: a case report. J Dermatol. 2012; 39:1035–1037.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download