Abstract
Background
Immunohistochemistry and polymerase chain reaction (PCR) are the most widely used methods for the detection of viruses. PCR is known to be a more sensitive and specific method than the immunohistochemical method at this time, but PCR has the disadvantages of high cost and skilled work to use widely. With the progress of technology, the immunohistochemical methods used in these days has come to be highly sensitive and actively used in the diagnostic fields.
Objective
To evaluate and compare the usefulness of immunohistochemistry and PCR for detection human papilloma virus (HPV) in wart lesions.
Methods
Nine biopsy samples of verruca vulgaris and 10 of condyloma accuminatum were examined. Immunohistochemical staining using monoclonal antibody to HPV L1 capsid protein and PCR were done for the samples. DNA sequencing of the PCR products and HPV genotyping were also done.
Results
HPV detection rate was 78.9% (88.9% in verruca vulgaris, 70.0% in condyloma accuminatum) on immunohistochemistry and 100.0% for PCR. HPV-6 genotype showed a lower positivity rate on immunohistochemistry (50.0%) as compared to that of the other HPV genotypes.
Conclusion
Immunohistochemistry for HPV L1 capsid protein showed comparable sensitivity for detection HPV. Considering the high cost and great effort needed for the PCR methods, we can use immunohistochemistry for HPV L1 capsid protein with the advantage of lower cost and simple methods for HPV detection.
Wart is a disease caused by infection with human papilloma virus (HPV), and it can be classified into several types like common wart, flat wart, plantar wart and genital wart according to the infection site and form of the lesion1. The diagnosis of wart is not difficult in a case that it shows a typical clinical pattern, but making the diagnosis may sometimes be difficult when it shows an atypical form, and in such a case wart is diagnosed by detecting HPV in the region.
In general, immunohistochemical staining and polymerase chain reaction (PCR) are used as the representative methods of virus detection2. The HPV detection rate of immunohistochemical staining in a wart lesion on the skin and mucous membrane was known to have very low sensitivity until the early 1990s, and in 1994 Wools et al.3 reported that only 20% of genital warts were positive for L1 capsid protein on immunohistochemical staining. Since then there has been no dermatological report of HPV detection in wart lesions using immunohistochemical staining. Yet with the advances of the technology to produce monoclonal antibodies as well as several techniques related to immunohistochemical staining, immunohistochemical staining for L1 capsid protein is now being actively used, without much concern over the sensitivity, for research and clinical procedures in the areas of obstetrics, gynecology, infectious diseases, otorhinolaryngology, etc. Particularly as large-scales studies on HPV have been recently conducted for the development of HPV vaccine, the convenience of immunohistochemical staining has become recognized and its use has been broadened456789. The PCR method is the most sensitive method and this is commonly used as a confirmatory test, but it has limitations to be widely used because it requires a lot of time, effort and cost2. PCR kits for the high-risk HPV genotypes that induce cervical cancer, etc. have been developed and they are being conveniently used in the field of obstetrics and gynecology, but these kits do not match the HPV genotypes that cause common dermatological diseases, so there are limitations to diagnose HPV disease in the area of dermatology with using the existing PCR kits.
Thus, this study attempted to detect HPV in common wart and genital wart specimens through immunohistochemical staining using monoclonal antibody to L1 capsid protein and the PCR method, which is now being commonly used, and to compare the results between the 2 methods. In addition, we identified genotypes by analyzing the base sequence of the PCR amplified product and we examined the difference in the positivity rate of immunohistochemical staining according to the genotype.
The objects of this study were 9 paraffin tissue slices obtained from 9 patients who were diagnosed with common wart clinically and histologically, and 10 paraffin tissue slices from 10 patients diagnosed with genital wart, and these patients were sampled from the patients who visited the Department of Dermatology of Seoul St. Mary's Hospital, the Catholic University of Korea. This study was approved by the Institutional Review Board of The Catholic University of Korea (IRB no. KC14ZISE0804).
Immunohistochemical staining was performed by the previously reported method and using a Cytoactiv HPV L1 Screening Set (Cytoimmun diagnostics GmbH, Pirmasens, Germany)4. The method is described briefly as follows. From the tissue slice fixed with paraffin, the paraffin was removed using xylene. In order to inactivate the endogenous peroxidase, the slice was put in PBS solution that contained 3% hydrogen peroxide and the slice was treated at room temperature for around 5 minutes. Protein blocking was performed using Ultra V Block (LabVision Corporation, Fremont, CA, USA). Then, the slice was treated with primary antibody diluted at 1:5 on a slide and this was allowed to react at room temperature for 60 minutes, and again the slice was treated with color former and this was allowed to react at room temperature for 50 minutes. The slice was then reacted with AEC substrate-chromogen solution (Dako, Copenhagen, Denmark) for 8 minutes and then it was counterstained with hematoxylin stain.
When only the nuclei were stained, the case was considered positive, and like the previously reported method, the degree of staining was graded to negative, 1+, 2+ and 3+4. That is, a case was graded as negative if the number of positively stained nuclei in the whole specimen was 0, 1+ if the number of positively stained nuclei in at least one high-power field was 1~3, 2+ if this was 4~10, and 3+ if this was 11 or greater.
DNA was isolated from a tissue slice fixed with paraffin and this was amplified by the PCR method. And a size of the amplified PCR products was examined through electrophoresis, and base sequence analysis was requested of Macrogen Inc. (Seoul, Korea) for HPV genotype identification. The detailed procedure was as follows.
In order to remove the paraffin from a 7 µm thick tissue slice embedded in paraffin and attached on a slide, the slice was treated with 100% xylene twice for 5 minutes each time. From the slide on which the paraffin was removed, the slice was separated through treatment with degradation buffer solution containing 0.25% NP-40 (5 mM MgCl2, 10 mM Tris-HCl, pH 7.4, 10 mM NaCl), and then it was moved to an Eppendorf tube. In order to remove the protein, buffer solution containing 0.5% sodiumdodecyl sulfate and proteinase K (final concentration 10 µg/ml) was added and the slice was put in a 56℃ constant-temperature water bath and reacted for 15~18 hours. After the degradation of the protein, the DNA was isolated by the phenol/chloroform and ethanol sedimentation method. The isolated DNA was melted in 1 ml TE buffer and kept at 4℃, and the amount of DNA was measured using an ultraviolet (UV) spectrophotometer (Shimadzu, Kyoto, Japan).
The prepared DNA specimen (10~200 ng) was put in a PCR tube of AccuPower PCR PreMix (Bioneer, Daejeon, Korea), and the HPV strain specific site was amplified using the degenerate primers CPI 5'-TTA TCW TAT GCC CAY TGT ACC AT-3' and CPIIG 5'-ATG TTA ATW SAG CCW CCA AAA TT-3'. Here, W expresses a base in which A or T is mixed together, Y for C or T, and S for C or G. As for the PCR conditions, initial denaturation was performed at 94℃ for 5 minutes and this was followed by denaturation at 94℃ for 1 minute for amplification, annealing at 56℃~60℃ for 1 minute and polymerization at 72℃ for 1 minute. After this procedure was performed for 40 cycles, the final extension was performed for 5 minutes. The PCR product was expected to be 187 bp, and it was electrophoresed on 1% agarose gel and stained with ethidium bromide, and the size of the PCR product was examined under UV light.
The base sequence analysis of the PCR product was requested of Macrogen Inc. for HPV genotype identification. The homology analysis of the found base sequence and the HPV genotype was performed using databases through the DNASIS and BLAST servers (GenBank, European Molecular Biology Laboratory, DNA Data Bank of Japan, and Protein Data Bank).
For the results of immunohistochemical testing using antibody to the HPV L1 capsid protein, 8 out of 9 common wart specimens were stained positively, and the other one was not stained, so the positive rate was 88.9%. The number of stained cells was 1+ in 6 out of the 8 positively stained specimens, and 3+ in the other 2.
Among the 10 genital wart specimens, 7 were stained positively and the other 3 were not stained, showing a positive rate of 70.0%. The number of stained cells was 1+ in 6 out of the 7 positively stained specimens and 2+ in the other.
For the result of detecting HPV by the PCR method, HPV was detected in all of the 9 common wart specimens and the 10 genital wart specimens for a detection rate of 100% (Table 1). When the genotype was identified through analyzing the base sequence of amplified PCR products, the genotypes HPV-2, HPV-6 and HPV-65 were identified in 2 specimens each, and HPV-7, HPV-18, and HPV-40 were identified in 1 each among the 9 common wart specimens. Coinfection of multiple genotypes was not observed in verruca vulgaris specimens. Among the 10 genital wart specimens, genotype HPV-6 was the most frequent and it was identified in 4 specimens, and HPV-11 was identified in 3 and HPV-7, HPV-33, and HPV-40 were identified in 1 each. Coinfection was not observed in genital wart specimens as well.
For the 9 common wart specimens, the HPV detection rate for immunohistochemical testing was 88.9% (8/9) and it was 100.0% (9/9) for the PCR method. For 1 specimen, HPV was identified by PCR, but a negative response was shown on immunohistochemical testing, and the genotype identified through PCR and base sequence analysis was HPV-6.
For the 10 genital wart specimens, the HPV detection rate of immunohistochemical testing was 70.0% (7/10), which was lower than that for the common wart specimens, and the HPV detection rate of PCR was 100.0% (10/10). For 3 specimens, HPV was identified by PCR but a negative response was shown on immunohistochemical testing, and the identified genotype was HPV-6 in 2 specimens and HPV-11 in 1.
Summing up the results for all of the common wart and genital wart specimens, the total HPV detection rate was 78.9% (15/19) for immunohistochemical testing and 100.0% (19/19) for the PCR method. For 4 specimens, HPV was identified by PCR, but a negative response was shown on immunohistochemical testing, and the identified genotype was HPV-6 in 3 specimens and HPV-11 in 1.
The positive rate of immunohistochemical staining was different according to the genotype identified by PCR, and the results are shown in Table 2. A notable result is that of the 6 specimens whose genotype was identified as HPV-6, only 3 were positive on immunohistochemical testing, showing a positive rate of 50.0%. Among the 3 specimens of HPV-11, 2 were positive in immunohistochemical test and the other was negative, showing a positive rate of 66.7% for immunohistochemical testing. In the specimens with other genotypes, the positive rate on immunohistochemical testing was 100%.
HPV is a virus belonging to papillomaviridae, and HPV induces warts in the human body and some genotypes cause malignant tumor. HPV is a virus with a double helix structure, and its diameter measures approximately 55 nm and over 100 different genotypes, according to the DNA sequence, have been found1. The genome of HPV can be divided into 8 open reading frames, which are the E1, E2, E4, E5, E6, and E7 genes that are involved with DNA replication and cell transformation, and the L1 and L2 genes that produce capsid protein.
The HPV genotypes, which are determined by the DNA sequence, can be classified by several methods. They are divided into the high-risk group and the low-risk group according to the carcinogenicity or into the cutaneous type and the genital-mucosal type according to the tissue of development1.
Our study focused on common wart and genital wart, which are commonly known as HPV infection diseases. The common HPV genotypes causing common wart are HPV-2, 4, 27, and 29 and rarely HPV-1, 6, 11, 16, 18, 31, 33~35, 39, 40, and 51~60, and other genotypes may cause the disease. The HPV genotypes common in genital wart include HPV-6, 11, 16, 18, 31, 33~35, 39, 40, and 51~60 and among them, HPV-6 and 11 belong to the low-risk group related to genital and cervical cancer1. In Japan, HPV-1a, HPV-4, HPV-65, HPV-27, HPV-2a, HPV-57b, HPV-16, and HVP-6a are frequently detected in common warts10. In Netherlands, HPV-27, HPV-57, and HPV-2 are most frequently observed in common warts11. In Korean, Moon et al.12 showed that HPV-6, HPV-11, and HPV-53 were frequently found in genital warts of HIV-positive patients. In this study, HPV-2, HPV-6, and HPV-65 are commonly detected in common warts, whereas HPV-6 and HPV-11 in genital warts.
It is not difficult to diagnose warts that show a typical pattern, but those warts showing an atypical clinical pattern are sometimes hard to differentiate from other diseases.
The diseases that may show a clinical pattern similar to wart include seborrheic keratosis, solar keratosis, nevus, pyogenic granuloma, and sebaceous hyperplasia. Squamous cell carcinoma is also known to show a form similar to wart. Lichen planus lesion may look like a flat wart, and acrokeratosis verruciformis and epidermolytic hyperkeratosis on the arms and legs may also be confused with wart. In the case of genital wart, the disease is known to be difficult to differentiate from lesions such as nevus, benign keratosis, cyst, an ectopic sebaceous gland, and syphilitic condyloma1.
In this way, the key point for differentiating wart from various other diseases is detecting HPV in the lesion, and the representative methods for proving HPV are immunohistochemical staining and PCR. Immunohistochemical staining for detecting HPV is usually performed using monoclonal antibody to HPV L1 capsid protein, and L1 capsid protein is known to be commonly expressed by most of the HPV genotypes13. In general, immunohistochemical staining is simpler and it requires less effort and cost than PCR and it can locate the site of expression, but it is less sensitive than PCR, so it may produce a false negative result. Furthermore, due to its low specificity, the specimen is stained non-specifically. PCR for HPV detection amplifies a particular site specific to HPV and the amplified product is examined through electrophoresis. It is used as a confirmatory test because of its high sensitivity and specificity, and it can even identify genotypes by analyzing the base sequence of the amplified DNA. Yet as compared to immunohistochemical staining, PCR has a limitation as a routine method because it requires a lot of effort and cost2.
Since immunohistochemical staining was reported to have very low sensitivity until the early 1990s, there have been few reports on immunohistochemical staining for wart lesions in the dermatological area3. On the contrary, immunohistochemical staining has been actively used in the area of obstetrics and gynecology as a tool for detecting HPV in various genital dysplasias, and many recent reports have been published without much concern over the method's sensitivity. This is probably because with the recent advances of technologies for producing various reagents and materials, for instance, the monoclonal antibody used in immunohistochemical staining, the sensitivity and specificity of immunohistochemical staining has been considerably improved and as a result, immunohistochemical staining produces much better results than the method in the early 1990s14. In this study as well, when HPV was detected through immunohistochemical staining, the rate of detecting HPV was 88.9% for common wart, 70% for genital wart and 78.9% in total. This result suggests that even with immunohistochemical staining, HPV can be detected with quite high sensitivity.
An interesting finding in this study is that HPV-2, 4, 27, and 29, which are known to be common in common warts, were found only in 2 specimens, which had the genotype HPV-2, out of the 9 common wart specimens used in this study, and the other 7 specimens had rare genotypes such as HPV-6, 7, 18, 40, and 65, which have been considered as pathogens of common wart. Among them, HPV-65 was found in 2 specimens, and it showed 83% gene agreement with HPV-4, which is commonly found in common warts15. Thus, it is likely that some of cases reported to be HPV-4 may actually have been HPV-65, and the genotypes that are common in Korea may be different from those reported in other countries.
Another finding is there were 4 cases in which PCR detected HPV, but the result of immunohistochemical staining was negative and 3 of the 4 cases were HPV-6. Of the 19 specimens, 6 were found to have the genotype HPV-6 and only 3 of them showed a positive response on immunohistochemical staining, that is, only 50% of the HPV-6 specimens were stained positively via immunohistochemical staining. The amino acid sequences of L1 proteins are similar in all HPV species. However, previous report showed that HPV-6 has type-specific epitopes16. As the monoclonal antibody used in this study was raised against HPV L1 capsid protein, it is possible that the antibody cannot recognize the HPV-6 type specific epitope. At the same time, it is difficult to fully interpret the results because the number of specimens was small in this study. Additional large-scale research may be required for more precise interpretation. We derived a limited conclusion that the positive rate of immunohistochemical staining may be lower for genital warts where the genotype HPV-6 is common, as compared to that for common warts.
From the results of this study, we confirmed that not only PCR but also immunohistochemical staining can detect HPV in common wart and genital wart lesions with good sensitivity. Thus, immunohistochemical staining, which is relatively simple and requires less effort and cost, may be widely applicable to prove the presence of HPV in verrucous lesions to differentiate wart from other diseases.
Figures and Tables
Fig. 1
Immunohistochemical staining for human papilloma virus L1 capsid protein. Positive staining in (A, B) verruca vulgaris and (C, D) condyloma accuminatum (A: ×40, B and D: ×200, C: ×100).
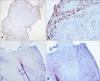
Table 1
Results of immunohistochemistry and PCR for detecting HPV
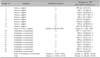
Table 2
Positivity of the immunohistochemical staining according to the human papilloma virus (HPV) genotypes

References
1. Androphy EJ, Lowy DR. Warts. In : Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill;2008. p. 1914–1923.
2. Swygart C. Human papillomavirus: disease and laboratory diagnosis. Br J Biomed Sci. 1997; 54:299–303.
3. Wools K, Bryan JT, Katz BP, Rodriguez M, Davis T, Brown DR. Detection of human papillomavirus L1 protein in condylomata acuminata from various anatomical sites. Sex Transm Dis. 1994; 21:103–106.

4. Negri G, Bellisano G, Zannoni GF, Rivasi F, Kasal A, Vittadello F, et al. p16 ink4a and HPV L1 immunohistochemistry is helpful for estimating the behavior of low-grade dysplastic lesions of the cervix uteri. Am J Surg Pathol. 2008; 32:1715–1720.

5. Rauber D, Mehlhorn G, Fasching PA, Beckmann MW, Ackermann S. Prognostic significance of the detection of human papilloma virus L1 protein in smears of mild to moderate cervical intraepithelial lesions. Eur J Obstet Gynecol Reprod Biol. 2008; 140:258–262.

6. Azzimonti B, Hertel L, Aluffi P, Pia F, Monga G, Zocchi M, et al. Demonstration of multiple HPV types in laryngeal premalignant lesions using polymerase chain reaction and immunohistochemistry. J Med Virol. 1999; 59:110–116.

7. Griesser H, Sander H, Hilfrich R, Moser B, Schenck U. Correlation of immunochemical detection of HPV L1 capsid protein in pap smears with regression of high-risk HPV positive mild/moderate dysplasia. Anal Quant Cytol Histol. 2004; 26:241–245.
8. Melsheimer P, Kaul S, Dobeck S, Bastert G. Immunocytochemical detection of HPV high-risk type L1 capsid proteins in LSIL and HSIL as compared with detection of HPV L1 DNA. Acta Cytol. 2003; 47:124–128.

9. Hilfrich R, Hariri J. Prognostic relevance of human papillomavirus L1 capsid protein detection within mild and moderate dysplastic lesions of the cervix uteri in combination with p16 biomarker. Anal Quant Cytol Histol. 2008; 30:78–82.
10. Hagiwara K, Uezato H, Arakaki H, Nonaka S, Nonaka K, Nonaka H, et al. A genotype distribution of human papillomaviruses detected by polymerase chain reaction and direct sequencing analysis in a large sample of common warts in Japan. J Med Virol. 2005; 77:107–112.

11. de Koning MN, Quint KD, Bruggink SC, Gussekloo J, Bouwes Bavinck JN, Feltkamp MC, et al. High prevalence of cutaneous warts in elementary school children and the ubiquitous presence of wart-associated human papillomavirus on clinically normal skin. Br J Dermatol. 2015; 172:196–201.

12. Moon SB, Moon SH, Park KJ. Detection and typing of human papillomavirus in anal condyloma acuminatum of HIV-positive patients. J Korean Surg Soc. 2010; 78:111–115.

13. Conway MJ, Meyers C. Replication and assembly of human papillomaviruses. J Dent Res. 2009; 88:307–317.

14. Molina-Ruiz AM, Santonja C, Rütten A, Cerroni L, Kutzner H, Requena L. Immunohistochemistry in the diagnosis of cutaneous viral infections--part I. Cutaneous viral infections by herpesviruses and papillomaviruses. Am J Dermatopathol. 2015; 37:1–14. quiz 12-14.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download