Abstract
Background
A small subset of adolescents atopic dermatitis (AD) tends to persist. This also leads to get more antibiotics exposure with advancing years. Antibiotic resistance has been regarded as a serious problem during Staphylococcus aureus treatment, especially methicillin-resistant S. aureus (MRSA).
Objective
It was investigated the S. aureus colonization frequency in the skin lesions and anterior nares of adolescent AD patients and evaluated the changes in S. aureus antimicrobial susceptibility for years.
Methods
Patients who visited our clinic from September 2003 to August 2005 were classified into group A, and patients who visited from August 2010 to March 2012 were classified into group B. To investigate the differences with regard to patients' age and disease duration, the patients were subdivided into groups according to age. Lesional and nasal specimens were examined.
Results
Among the 295 AD patients, the total S. aureus colonization rate in skin lesions was 66.9% (95/142) for group A and 78.4% (120/153) for group B. No significant changes in the systemic antimicrobial susceptibilities of S. aureus strains isolated from adolescent AD patients were observed during about 10-year period. The increased trend of MRSA isolation in recent adolescent AD outpatients suggest that the community including school could be the source of S. aureus antibiotic resistance and higher fusidic acid resistance rates provides evidence of imprudent topical use.
Atopic dermatitis (AD) is a genetically determined, chronically relapsing inflammatory skin disease with multiple pathogenic factors. While AD occurs most commonly during infancy and children, a smaller subset of adolescents has persistent or new-onset AD. The association between Staphylococcus aureus infection and AD is well demonstrated by many investigators12. S. aureus can be found from dermatitic lesions of more than 90% of patients with AD, but also from approximately 70% taken from unaffected areas123. S. aureus plays an important role as a triggering factor34. The relationship between AD and exacerbation mechanism by S. aureus is mainly due to the superantigens (sAgs) and sAgs-specific immunoglobulin E that stimulate various numbers of different T-cell clones and cytokine secretion. It has been often proposed that bacterial skin infections are uncommon in AD, while AD patients are commonly colonized with S. aureus. Recently lesional S. aureus colonization correlates positively with AD clinical severity, and anti-staphylococcal antibiotic therapy can reduce the severity of AD characteristic inflammation56.
The anterior nares are an important S. aureus colonization reservoir. High rate (75%~90%) of nasal carriage of S. aureus has been reported in adults and children with AD. In contrast, nasal colonization has ranged from 10% to 50% in normal controls. Thus S. aureus carriage in the nose should be targeted for decolonization78.
Antibiotic resistance is increasing around the world to date and has been regarded as an important issue during S. aureus treatment since a long time ago, especially methicillin-resistant S. aureus (MRSA). MRSA is more difficult to treat because it is resistant to a number of widely used antibiotics. The increasing incidence of community-acquired MRSA (CA-MRSA) in skin infections presents major challenges in the treatment direction. It also raises concerns that the eczematous skin lesions of AD patients might be favorable CA-MRSA reservoirs910. Topical fusidic acid and mupirocin have been commonly prescribed to eradicate different skin infections via over-the-counter pharmacy in Korea. Although many Asian countries have high MRSA infection rates, there have been no publications about changes in the prevalence of antibiotic-resistant S. aureus, including CA-MRSA, in AD patients. Moreover, few studies have dealt with adolescent AD about S. aureus colonization and its susceptibilities to various antibiotics.
In present study, it was investigated the S. aureus colonization frequency in the skin lesions and anterior nares of AD patients and evaluated the changes in S. aureus antimicrobial susceptibility for years. Differences were also analyzed with regard to patient age and disease duration. Also, we investigated the prevalence of topical fusidic acid- and mupirocin-resistant S. aureus in adolescent AD.
Adolescent AD patients with no evidence of skin infection, who visited the outpatient clinic of the Department of Dermatology, Pusan National University Hospital (Busan, Korea), were enrolled in the study. AD was diagnosed according to the Hanifin and Rajka diagnostic criteria11. Total 295 patients who initially visited our clinic from September 2003 to August 2005 were classified into group A, and patients who initially visited from August 2010 to March 2012 were classified into group B. To investigate the differences with regard to patient age and disease duration, the patients were subdivided into groups according to age (younger than 18 years and older than 18 years) and disease duration (less than 1 year, 1~5 years, and more than 5 years). At the first visit, the patient age and disease duration were estimated and AD severity was assessed according to the SCORing Atopic Dermatitis (SCORAD) index12. The exclusion criteria were the presence of other skin or allergic diseases; a recent (within 4 weeks) history of inpatient hospital admission; recent (within 4 weeks) treatment with antibiotics, systemic corticosteroids, or immunosuppressants; and treatment with topical antibiotics in the previous 2 weeks.
The study protocol was approved by the Pusan National University Hospital Institutional Review Board (IRB no. 1409-012-035). Lesional skin specimens were obtained by rolling sterile cotton-tipped swab sticks (transport medium swab; Micromedia Co., Seoul, Korea) over the most affected skin areas twice for at least 5 seconds each. Nasal swabs were obtained by reaching upward toward the top of both anterior nares with sterile cotton-tipped swab stick, followed by a 360° twist to sweep the entire vestibule. The swab specimens were immediately placed in Amie's medium (Micromedia Co.) and were streaked on sheep blood agar plates (Asan Medical Co., Seoul, Korea), incubated at 35℃, and examined at 24 and 48 hours. Colonies were identified in a blind manner by other investigator. In some selected samples of two groups, antibiotic susceptibility tests were performed with the same Vitek 2 system (BioMérieux, Durham, NC, USA) according to the manufacturer's instructions.
For group A, a panel of 8 antibiotics (clindamycin, erythromycin, habekacin, oxacillin, gentamicin, penicillin, bactrim, and vancomycin) was used to test for gram-positive bacteria. For group B, a panel of 11 antibiotics or combinations (ciprofloxacin, fusidic acid, rifampin, teicoplanin, tetracycline, nitrofurantoin, quinupristin/dalfopristin, linezolid, telithromycin, mupirocin, and tigecycline) was added to the previous panel.
The Shapiro-Wilk normality test was performed to evaluate differences between the groups with regard to patient age, disease duration, and severity, using the Predictive Analytics Software package (PASW for Windows; IBM Co., Armonk, NY, USA). The chi-square and Fisher's exact tests were performed to estimate differences in the colonization rates between groups that were subdivided according to time period, age, and disease duration. Statistical significance was defined as a p-value of <0.05.
The clinical and demographic data for groups A and B are presented in Table 1. Overall, 142 and 153 patients were enrolled in groups A and B, respectively. The mean patient age in group A was 13.7 years, and the mean disease duration was 7.3 years. The mean patient age in group B was 18.3 years, and the mean disease duration was 10.0 years. The difference in clinical severity according to the SCORAD index was not significant between the 2 groups.
Among the 295 AD patients, the total S. aureus colonization rate in skin lesions was 66.9% (95/142) for group A and 78.4% (120/153) for group B (p=0.03). In group A, 142 samples were taken from the nares. In group B, 97 sampling were done in the nares. In the nasal swabs, S. aureus was found to colonize 64.1% (91/142) of the group A patients and 63.9% (62/97) of the group B patients (p=0.05). To analyze antibiotics sensitivity, 64 samples and 57 samples were used at the lesion and nares in group A. One hundred and six samples and 23 samples were used in each of group B.
In group A, 2 of 64 (3.1%) and 4 of 57 (7.0%) patients carried MRSA in the lesional skin and the anterior nares, respectively, whereas in group B, 11 of 106 (10.4%) and 3 of 23 (13.0%) patients carried MRSA in the lesional skin and the anterior nares, respectively (p>0.05; Table 2).
Changes in S. aureus antimicrobial susceptibility in adolescent AD patients over time are shown in Table 3. In lesional skin, the rates of S. aureus susceptibility to clindamycin and erythromycin increased significantly in group B when compared with those in group A. The rates of susceptibility of S. aureus in lesional skin to fusidic acid and mupirocin, which are the main topical agents used to treat AD skin infections, were 67.0% and 95.3%, respectively in group B. The susceptibility rates of S. aureus in the anterior nares to fusidic acid and mupirocin were 60.9% and 91.3%, respectively. The rate of susceptibility of S. aureus in nasal swabs to erythromycin increased significantly during about 10-year period. For samples of both lesional skin and anterior nares, the penicillin susceptibility rate increased over time but remained much lower than those for other antibiotics.
The MRSA colonization rates did not significantly differ between groups A and B (p>0.05). All isolated MRSA strains were susceptible to habekacin, bactrim, and vancomycin (Table 4).
S. aureus antimicrobial susceptibility was also analyzed according to the patient age (younger than 18 years versus older than 18 years; Table 5). In patients older than 18 years, S. aureus in samples of both the lesional skin and anterior nares was significantly more susceptible to erythromycin, compared to the susceptibility in patients younger than 18 years. Low susceptibility rates to penicillin and fusidic acid were observed regardless of the patient age.
Table 6 shows the antimicrobial susceptibility of S. aureus with regard to the disease duration. For lesional skin samples, erythromycin susceptibility rate was significantly lower in patients with disease duration of less than 5 years than in those with disease duration of more than 5 years. Low susceptibility rates to penicillin and fusidic acid were observed regardless of the disease duration.
S. aureus may not be of primary importance in AD pathogenesis but is an important triggering and/or aggravating factor in cutaneous AD inflammation due to S. aureus sAgs13. S. aureus colonization rate is significantly higher in AD patients than in normal controls because the stratum corneum of AD patients is highly susceptible to colonization by various bacteria including S. aureus1014. Also, several studies have showed that AD keratinocytes produce lower amounts of antimicrobial peptides and this may increase the colonization and infection with S. aureus15. This study revealed the increasing prevalence of S. aureus colonization in adolescent AD skin (from 66.9% to 78.4% [p=0.03]) during about 10-year period. This is consistent with the increased S. aureus colonization rates over time that were previously described in cross-sectional studies1016. The increased S. aureus colonization rate indicates the importance of determining the antibiotic susceptibility of S. aureus and controlling AD inflammations effectively.
Prolonged or imprudent antibiotic use may induce the development of antibiotic-resistant S. aureus strains1317. Although careful antibiotic use has been often suggested, to our knowledge, no reports have investigated changes in antibiotics susceptibility only in adolescent AD patients. Diamantis et al.18 reported a comparison of antibiotic resistance patterns in pediatric dermatology patients infected by S. aureus in 2005~2007 (66% of children with AD) versus 2008~2009 (72.4% with AD). They found an increase in S. aureus antibiotic resistance except to methicillin, which surprisingly decreased. Other pediatric dermatology clinic in North Carolina also conducted the antibiotic susceptibility profiles in S. aureus cutaneous infections between 2005 and 200719. The subjects of study included 66% of AD patients, and they demonstrated the following resistance patterns: penicillin (86%), erythromycin (46%), methicillin (32%), clindamycin (22%), gentamicin (3%), vancomycin (0%), and trimethoprim-sulfame-thoxazole (0%).
In the patients with CA-MRSA infections, traditional MRSA risk factors are absent and resistance is usually limited to β-lactam antibiotics16. Following the first report in 1961 in England, the incidence of MRSA has increased progressively20. Recently, MRSA infections have been described in patients without established risk factors who are living in the community, especially AD patient. In a previous report, 4.2% of those obtained from the general outpatient pediatric population showed methicillin resistance and in our study, the frequency of CA-MRSA-positive skin samples was 10.4% despite smaller samples2122.
This agrees with earlier findings that the CA-MRSA prevalence was 7.4% to 18.4% of skin cultures from AD patients910. In our study, increased trend of MRSA isolation rates were observed in both skin lesions and anterior nares although these increases were statistically insignificant. Moreover, MRSA colonization rates in healthy individuals were reported as 0%~9% in previous studies, and these rates are comparable with those of AD patients212223. Since S. aureus has a predilection for damaged skin and AD patients are frequently exposed to antimicrobials, the relatively lower rate of MRSA colonization observed in our study might be meaningful. But, increased caution during MRSA infection management is required in AD patients, as they can be sources of CA-MRSA. All MRSA strains in this study were susceptible to vancomycin, the treatment of choice for MRSA infections. However, vancomycin use should be reserved for MRSA infections which is based by culture.
The present study verified the low rates of S. aureus penicillin susceptibility in adolescent AD outpatients (14.0%~26.1%), regardless of the time period, age, or disease duration. This finding is consistent with a previous study, which reported that 13% of AD patients were sensitive to penicillin16. The penicillin susceptibility rate remains much lower than that of other antibiotics, even though penicillin usage is restricted in AD patients in Korea. This is because the rates of declining resistance appear to be slower than that of emerging resistance and appear to vary with different agent classes24.
Fusidic acid has been widely used as topical antimicrobial to treat bacterial superinfections in AD patients until now. In our study, relatively low susceptibility rates (60.9%~67.0%) to fusidic acid were observed regardless of the patient age and disease duration. In Korea, topical fusidic acid antimicrobial has been classified over-the counter drug and its low susceptibility provides evidence of imprudent topical use. Our results suggest that another agent should be used for the treatment of adolescent AD patients with suspected S. aureus infections. In a British study published in 2009, 41% of S. aureus isolates from dermatology patients were fusidic acid-resistant, compared with a 50% resistance rate in 2001, due to usage restrictions and a significant decrease in the use of topical fusidic acid2526. The authors supposed that a lag period might occur before fusidic acid resistance is absent from the community. Topical fusidic acid use should be restricted due to the current high level of resistance. A relatively high susceptibility (91.3%~95.3%) to mupirocin was demonstrated in our study, regardless of the time period, patient age, and disease duration. This finding was consistent with previous results, which suggested that 4% of isolates from AD patients were mupirocin-resistant27. These results indicate that topical fusidic acid has been used more extensively than mupirocin. However, the potential for the development of bacterial resistance to mupirocin ointment should not be ignored, and thus caution regarding its use is needed to retain the high antimicrobial effects2829.
Despite the concerns of many dermatologists, there were no significant changes in S. aureus antimicrobial susceptibility in AD, except for erythromycin and clindamycin, during the recent 10-year period. In the 1990s, erythromycin was the first-line treatment for bacterial infections in AD patients, but its use has decreased after reports of high erythromycin resistance rates in S. aureus and recommended usage restrictions3031. According to our data, the erythromycin susceptibility rate increased significantly during the period from 59.4% to 91.5% for the lesional skin samples and from 63.2% to 91.3% for the anterior nare samples. Previous reports conducted in the USA, Europe, and Asia indicated that 51%~76% of S. aureus strains were erythromycin-susceptible in 1999323334. Hoeger's study35 of antimicrobial susceptibility, which was published in 2004, revealed that the rate of S. aureus erythromycin resistance remained low in 82% of AD patients. Thus, erythromycin should no longer be recommended as a therapeutic agent for S. aureus-infected AD patients.
In previous studies conducted in 1997 and 1999, a 91%~97% clindamycin susceptibility rate was demonstrated in Singapore and Europe3637. In 2008, Niebuhr et al.30 reported that clindamycin has been recommended as a first-line therapy (alternative to cephalexin or cefuroxime) and for staphylococcal skin infections in Germany, and another study conducted in 2005 and 2006 revealed a clindamycin susceptibility rate of 79% for S. aureus3536. Interestingly, in our study, the clindamycin susceptibility rate in lesional skin samples increased significantly from 81.3% to 94.3% during the period. In Korea, reports described relatively low S. aureus susceptibility rates to clindamycin (48%~82.6%) and recommended clindamycin usage restrictions; our data reflect those efforts3738. Clindamycin acts against a variety of anaerobic bacteria, but broad antibiotic coverage is not required in AD, as S. aureus is the most frequent skin infection-inducing microorganism30. Therefore, clindamycin should also no longer be suggested as a therapeutic agent for S. aureus infections in AD patients.
According to our age-based analysis, for both the lesional skin and nasal cultures, patients younger than 18 years have significantly lower susceptibility rates against erythromycin than do patients older than 18 years. Our results are comparable to those of Arkwright et al.39, who studied age-related changes in the S. aureus prevalence on affected AD skin. The authors found that children older than 5 years had a higher prevalence of erythromycin-resistant S. aureus (35%) than did younger children (26%). This discordance of results between the 2 studies might be due to differences in the age groups and distributions. In our study, the group of patients who were younger than 18 years included more group A patients with significantly higher erythromycin resistance rates. According to our disease duration-based analysis, different resistant patterns have shown in various antimicrobials, although we did not find a statistic difference. In our population, patients with disease duration of less than 5 years were less susceptible to erythromycin than patients with disease duration of more than 5 years. It was possible that erythromycin was no more used as a therapeutic agent for S. aureus-infected AD patients in different outpatient settings. A previous study by Ewing et al.40 supported the idea that antibiotic therapy is not helpful in AD patients who do not present signs of bacterial infection. Moreover, the MRSA incidence rate increased after a 4-week systemic antibiotic therapy course. Continuous antibiotic use with the intent to clear S. aureus colonization in AD may ultimately result in the failures of these antibiotics to treat severe infections, which are not uncommon in AD41. The therapeutic recommendation for bacterial infections in AD patients includes a combination therapy of topical anti-inflammatory drugs and topical/systemic antibiotics during the early stage when clinical signs of a secondary bacterial infection are present42. Recently, cephalexin, a first-generation cephalosporin, was found to be a good first-line antibiotic for the treatment of secondary S. aureus infections in AD due to its restricted antimicrobial spectrum, which comprises gram-positive bacteria and a limited number of gram-negative strains30.
In conclusion, despite our concerns, no significant changes in the antimicrobial susceptibilities of S. aureus strains isolated from AD patients were observed during a 10-year period. These results indicate that in medical society, a high level of attention is focused on the misuse and abuse of antibiotics. However, the increased trend of MRSA isolation and fusidic acid resistance rates in recent AD outpatients suggest that the community including school could be the source of S. aureus antibiotic resistance and its imprudent prescription. To appropriately treat skin infections in adolescent AD, proper antibiotic use through periodic reviews and understandings of changes in microorganisms and antimicrobial sensitivities is necessary to avoid the excessive use of broad-spectrum empiric antibiotics.
Figures and Tables
Table 1
Demographics of atopic dermatitis patients

Table 2
Total colonization rates (%) of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in lesional skin and anterior nares

Table 3
Change in Staphylococcus aureus antimicrobial susceptibility
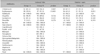
Table 4
Change in methicillin-resistant Staphylococcus aureus antimicrobial susceptibility

Table 5
Staphylococcus aureus antimicrobial susceptibility with respect to age in patients with atopic dermatitis
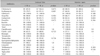
Table 6
Staphylococcus aureus antimicrobial susceptibility with respect to disease duration in patients with atopic dermatitis

References
1. Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977; 113:780–782.

3. Roll A, Cozzio A, Fischer B, Schmid-Grendelmeier P. Microbial colonization and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004; 4:373–378.

4. Kim BS, Kim JY, Lim HJ, Lee WJ, Lee SJ, Kim JM, et al. Colonizing features of Staphylococcus aureus in early childhood atopic dermatitis and in mothers: a cross-sectional comparative study done at four kindergartens in Daegu, South Korea. Ann Allergy Asthma Immunol. 2011; 106:323–329.

5. Williams JV, Vowels BR, Honig PJ, Leyden JJ. S. aureus isolation from the lesions, the hands, and the anterior nares of patients with atopic dermatitis. Pediatr Dermatol. 1998; 15:194–198.

6. Kim BS, Park JY, Song CH, Kim JY, Lim HJ, Lee HS, et al. Clarifying the transmission route of Staphylococcus aureus colonizing the skin in early childhood atopic dermatitis. Ann Allergy Asthma Immunol. 2012; 109:448–453.

7. Herz U, Bunikowski R, Renz H. Role of T cells in atopic dermatitis. New aspects on the dynamics of cytokine production and the contribution of bacterial superantigens. Int Arch Allergy Immunol. 1998; 115:179–190.
8. Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. Br J Dermatol. 1988; 119:189–198.

9. Chung HJ, Jeon HS, Sung H, Kim MN, Hong SJ. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from children with eczematous atopic dermatitis lesions. J Clin Microbiol. 2008; 46:991–995.

10. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009; 123:e808–e814.

11. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
12. Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997; 195:10–19.

13. Taskapan MO, Kumar P. Role of staphylococcal superantigens in atopic dermatitis: from colonization to inflammation. Ann Allergy Asthma Immunol. 2000; 84:3–10. quiz 11-12.

14. Sung HC, Jung HD, Park KD, Lee WJ, Lee SJ, Kim DW. A quantitative culture study of Staphylococcus aureus in adolescent and adult patients with atopic dermatitis using the contact-plate sampling technique. Korean J Dermatol. 2007; 45:673–679.
15. Donald YML, Lawrence FE, Mark B. Atopic dermatitis (Atopic eczema). In : Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. New York: McGraw-Hill Medical;2012. p. 168.
16. Goh CL, Wong JS, Giam YC. Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the National Skin Centre, Singapore. Int J Dermatol. 1997; 36:653–657.

17. Ellis MW, Lewis JS 2nd. Treatment approaches for community-acquired methicillin-resistant Staphylococcus aureus infections. Curr Opin Infect Dis. 2005; 18:496–501.

18. Diamantis ML, Ortega-Loayza AG, Morrell DS. Update on the characterization of Staphylococcus aureus skin infections in a pediatric dermatology tertiary health care outpatient facility: antibiotic susceptibility patterns and decreased methicillin resistance. J Am Acad Dermatol. 2011; 64:440–441.

19. Ortega-Loayza AG, Diamantis SA, Gilligan P, Morrell DS. Characterization of Staphylococcus aureus cutaneous infections in a pediatric dermatology tertiary health care outpatient facility. J Am Acad Dermatol. 2010; 62:804–811.

20. Eriksen KR. "Celbenin"-resistant staphylococci. Ugeskr Laeger. 1961; 123:384–386.
21. Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005; 352:1436–1444.

22. Matiz C, Tom WL, Eichenfield LF, Pong A, Friedlander SF. Children with atopic dermatitis appear less likely to be infected with community acquired methicillin-resistant Staphylococcus aureus: the San Diego experience. Pediatr Dermatol. 2011; 28:6–11.

23. Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics. 2008; 121:1090–1098.

24. Hsueh PR. Decreasing rates of resistance to penicillin, but not erythromycin, in Streptococcus pneumoniae after introduction of a policy to restrict antibiotic usage in Taiwan. Clin Microbiol Infect. 2005; 11:925–927.

25. Mitra A, Mohanraj M, Shah M. High levels of fusidic acid-resistant Staphylococcus aureus despite restrictions on antibiotic use. Clin Exp Dermatol. 2009; 34:136–139.

26. Shah M, Mohanraj M. High levels of fusidic acid-resistant Staphylococcus aureus in dermatology patients. Br J Dermatol. 2003; 148:1018–1020.

27. Kedzierska A, Kapińska-Mrowiecka M, Czubak-Macugowska M, Wójcik K, Kedzierska J. Susceptibility testing and resistance phenotype detection in Staphylococcus aureus strains isolated from patients with atopic dermatitis, with apparent and recurrent skin colonization. Br J Dermatol. 2008; 159:1290–1299.
28. Jones JC, Rogers TJ, Brookmeyer P, Dunne WM Jr, Storch GA, Coopersmith CM, et al. Mupirocin resistance in patients colonized with methicillin-resistant Staphylococcus aureus in a surgical intensive care unit. Clin Infect Dis. 2007; 45:541–547.

29. Park SY, Kim SM, Park SD. The prevalence, genotype and antimicrobial susceptibility of high- and low-level mupirocin resistant methicillin-resistant Staphylococcus aureus. Ann Dermatol. 2012; 24:32–38.

30. Niebuhr M, Mai U, Kapp A, Werfel T. Antibiotic treatment of cutaneous infections with Staphylococcus aureus in patients with atopic dermatitis: current antimicrobial resistances and susceptibilities. Exp Dermatol. 2008; 17:953–957.

31. Park CW. Pharmacologic treatment of atopic dermatitis. J Korean Med Assoc. 2006; 49:1046–1053.
32. Sugeng MW, Ang P, Tan HH, Goh CL. Characteristics of bacterial skin infections in children compared to adults at a tertiary dermatologic center. Int J Dermatol. 1999; 38:582–586.

33. Jones ME, Schmitz FJ, Fluit AC, Acar J, Gupta R, Verhoef J. Frequency of occurrence and antimicrobial susceptibility of bacterial pathogens associated with skin and soft tissue infections during 1997 from an International Surveillance Programme. SENTRY Participants Group. Eur J Clin Microbiol Infect Dis. 1999; 18:403–408.

34. Doern GV, Jones RN, Pfaller MA, Kugler KC, Beach ML. Bacterial pathogens isolated from patients with skin and soft tissue infections: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). SENTRY Study Group (North America). Diagn Microbiol Infect Dis. 1999; 34:65–72.

35. Hoeger PH. Antimicrobial susceptibility of skin-colonizing S. aureus strains in children with atopic dermatitis. Pediatr Allergy Immunol. 2004; 15:474–477.

36. Schöfer H, Brockmeyer N, Dissemond J, Effendy I, Esser S, Geiss HK, et al. Staphylococcal infections of the skin and mucous membranes. Guideline of the German Dermatologic Society, Study Group of Dermatologic Infectiology. J Dtsch Dermatol Ges. 2005; 3:726–734.
37. Bae EY, Lee JD, Cho SH. Isolation of causative microorganism and antimicrobial susceptibility in impetigo. Korean J Dermatol. 2003; 41:1278–1285.
38. Hong HJ, Lee CH, Park CO, Jung IW, Lee SH, Ko KS, et al. A clinical study on staphylococcus aureus bacteremia. Korean J Med. 1997; 53:359–370.
39. Arkwright PD, Daniel TO, Sanyal D, David TJ, Patel L. Age-related prevalence and antibiotic resistance of pathogenic staphylococci and streptococci in children with infected atopic dermatitis at a single-specialty center. Arch Dermatol. 2002; 138:939–941.

40. Ewing CI, Ashcroft C, Gibbs AC, Jones GA, Connor PJ, David TJ. Flucloxacillin in the treatment of atopic dermatitis. Br J Dermatol. 1998; 138:1022–1029.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download