Abstract
Background
Cutaneous pustular disorders include generalized pustular psoriasis (GPP) and acute generalized exanthematous pustulosis (AGEP).
Objective
To identify differences between GPP and AGEP, here we immunohistochemically evaluated interleukin (IL)-36 and the IL-23/Th17 axis.
Methods
This retrospective comparative immunohistochemical study was completed using 11 biopsies of 11 cases of GPP and 11 biopsies of 11 cases of AGEP. Through staining with the anti-IL-36-alpha (IL-36α), anti-IL-36 receptor antagonist (IL-36Ra), anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), anti-IL-23, anti-IL-17, and anti-IL-8 antibodies, main expression location and intensity were visualized in the epidermis and dermis.
Results
In both diseases, diffuse IL-36α expression was observed in the epidermis. IL-36Ra expression was observed in the dermal perivascular area as well as in the epidermis. NF-κB expression was observed in the epidermis and perivascular dermal area. Diffuse IL-23 and IL-17 expression was seen in the whole epidermis and the perivascular dermal area. IL-8 was expressed in the subcorneal pustules and parakeratotic area. Contrary to other cytokines, IL-23 expression in the epidermis of patients with GPP was more intense than only that in patients with AGEP.
Generalized pustular psoriasis (GPP) is a rare variant of psoriasis that can be fatal; therefore, systemic treatment and supportive care should be required12. Acute generalized exanthematous pustulosis (AGEP) is a cutaneous reaction to medications, infection, and even foods34. GPP and AGEP share common clinicopathological features characterized by widespread pustules with or without systemic symptoms such as fever, malaise, and leukocytosis156. Due to clinical similarities such as extensive pustules and systemic symptoms in two diseases, differentiation between the two diseases is quite difficult for physicians in the clinical setting. Despite several histological and immunohistochemical trials to identify differences between the two pustular disorders, no decisive differential features have been demonstrated67.
Although the pathophysiology of each disorder remains elusive and complicated, several cytokines such as interleukin (IL)-8, IL-17, IL-23, and IL-36, which are related to both disorders, have recently been investigated8910111213. However, comparative immunohistochemical studies between GPP and AGEP are relatively few7. Mutations of the gene for IL-36 receptor antagonist (IL-36Ra) were recently reported in patients with GPP and AGEP14151617. Such findings support the idea that a common pathomechanism might exist in the two diseases.
Therefore, here we aimed to use immunohistochemical methods to demonstrate and compare IL-36 family expression in GPP and AGEP. We also investigated key cytokines focused on the IL-23/Th17 axis, which is commonly involved in both GPP and AGEP.
A retrospective review of the clinical and histological data of patients with GPP and AGEP between 2002 and 2013 was conducted in Department of Dermatology, Ajou University Hospital, Suwon, Korea. The diagnosis of each disorder was based on previous reports618. The diagnosis of GPP was based on clinical courses, clinical photographs, and histological features exhibiting characteristic spongiform pustules61819. Diagnosis of AGEP was evaluated according to the validation system of Sidoroff et al.18. The clinical and immunohistochemical characteristics were investigated in GPP and AGEP using medical records, clinical photographs, histological slides. This study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-KSP-12-425).
Several parameters were reviewed including sex ratio, mean age, disease duration, antecedent events before pustular eruptions, previous history of psoriasis vulgaris, treatment modalities against pustular attacks, and recurrence using data from the medical records and clinical photographs.
Using formalin-fixed, paraffin-embedded specimens, hematoxylin and eosin and immunohistochemical staining were performed using antibodies against IL-36α(R&D Systems, Minneapolis, MN, USA), IL-36Ra (R&D Systems), nuclear factor kappa-light-chain-enhancer of activated B cells p65 (NF-κB; Abcam, Cambridge, UK), IL-23 (Biolegend, San Diego, CA, USA), IL-17 (Abcam), and IL-8 (Abcam). The immunohistochemical results were analyzed individually in the epidermis and dermis using Image Pro Plus Version 4.5 (Media Cybernetics Co., Silver Spring, MD, USA). The expressions of each immunohistochemical staining were determined by ratio of the stained area to measured epidermal area and dermal area on the representative area of each specimen as a blinded state. Dermal area was defined as the area within 200 µm from epidermo-dermal junction and representative area was defined as the area represents average stained area in consideration of overall tendency at each specimen. To determine the expression of NF-κB positivity, the ratio of NF-κB-positive cell count to the overall cell count within the epidermis or dermis was determined. For the NF-κB staining, positive findings were defined as strong nuclear staining, easily observed at the scanning magnification. For each frame, the tracing was repeated 3 times and the mean was determined.
Using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA), statistical analyses were performed using the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. Data were expressed as mean values and their standard deviation. Values of p<0.05 were considered statistically significant.
Twenty-two patients visited the institution for the pustular eruptions. Based on the diagnostic definition mentioned above, 11 patients were diagnosed with GPP and 11 with AGEP. The patients' clinical characteristics are summarized in Table 1. The mean disease duration was 590.2 days in patients with GPP and 4.2 days in patients with AGEP. Predisposing factors or antecedent events to pustular eruptions were reported to vary and include medication (antibiotics or antifungal agents), viral infection (upper respiratory infection or hemorrhagic febrile renal syndrome), pregnancy, and even food ingestion (Sumac chicken). The main treatment for each disease was systemic corticosteroids. A total of six patients with GPP and nine patients with AGEP received systemic corticosteroid treatment followed by clinical improvement. Four patients with GPP experienced clinical recurrence versus none of the patients with AGEP.
Immunohistochemical staining using IL-36α, IL-36Ra, NF-κB, IL-23, IL-17, and IL-8 was performed. Expression location and intensity were analyzed individually in the epidermis and dermis (Table 2, Fig. 1). In GPP and AGEP, diffuse IL-36α expression was observed in the epidermal area, and their degrees of intensity were similar (0.19 in GPP vs. 0.27 in AGEP, p=0.11). IL-36Ra expression also showed similar patterns with that of IL-36α at the epidermal level in the two groups (0.25 in GPP vs. 0.30 in AGEP, p=0.45). Furthermore, IL-36Ra was expressed in the perivascular dermal area with no difference between the two groups (0.03 in GPP vs. 0.03 in AGEP, p=0.72). Diffuse NF-κB expression was observed throughout the epidermis and the perivascular dermal area; however, there was no significant differences in two groups (0.48 in GPP vs. 0.48 in AGEP, p=0.82 in the epidermis; 0.03 in GPP vs. 0.04 in AGEP, p=0.34 in the dermis). IL-23 was mainly expressed in the epidermis and the perivascular dermal area in both groups with a significant difference only in the epidermis (0.60 in GPP vs. 0.44 in AGEP, p<0.05 in the epidermis; 0.07 in GPP vs. 0.04 in AGEP, p=0.08 in the dermis). IL-17 expression was also observed in the epidermis and perivascular dermal area but did not differ significantly between the two groups (0.29 in GPP vs. 0.22 in AGEP, p=0.45 in the epidermis; 0.05 in GPP vs. 0.05 in AGEP, p=0.38 in the dermis). IL-8 was predominantly expressed in the epidermis, especially in the pustular and parakeratotic areas in the two groups. However, the intensity did not differ significantly between the two groups (0.08 in GPP and 0.07 in AGEP, p=0.82).
The histological or clinical differentiation between GPP and AGEP is problematic, and several studies have failed to report any clear characteristics6. In addition, various cytokines related to each disorder were recently investigated11131620. The principal cytokines were the IL-36 family and IL-23/Th17 axis associated cytokines.
IL-36 and IL-36Ra are members of the IL-1 family and have been reported in psoriasis, especially pustular psoriasis. IL-36 is overexpressed in psoriatic lesional skin1220. On the other hand, mutations of the gene for IL-36Ra were reported in patients with intractable and severe GPP141517. Studies of IL-36 and its antagonist in patients with AGEP have also been reported16. In both diseases, diffuse uptake in the epidermal or perivascular dermal area was noted without significant differences. This finding suggests that the roles of IL-36α and IL-36Ra are similar in the two diseases. In addition, IL-36 activates inflammatory pathways such as the NF-κB pathway21. Therefore, we compared the expression of NF-κB in GPP and AGEP and observed it in the entire epidermal and perivascular dermal areas in both groups with no significant differences.
The IL-23/Th17 axis is a recently well-defined psoriasis pathogenesis that has been investigated in various inflammatory disorders because it is widely involved in their pathogenesis; therefore, it is considered as a potential treatment target102223. IL-23, which is produced by activated dendritic cells, stimulates T cells, which results in increased IL-17 production. The functions of IL-17 are synergized with IL-23, followed by localized skin inflammation11. The detection of increased IL-17–positive T lymphocytes in AGEP was reported assisting that T helper 17 cells or IL-17 might contribute to local inflammation in both psoriasis and AGEP1324. In our study, IL-23 and IL-17 expressions were observed in the epidermis and perivascular dermal area in both diseases. IL-23 expression in the epidermis of patients with GPP was more intense than that in patients with AGEP. These findings suggest that the expression of IL-23 might play a role in the differentiation between GPP and AGEP.
IL-8 is a chemokine that is secreted by various cells including epithelial cells and macrophages. Its functions include chemotaxis of neutrophils and lymphocytes and the induction of epidermal proliferations82526. IL-8 is also released from T lymphocytes, which results in pustule formation in AGEP, while IL-8–producing cells are involved in the pathogenesis of neutrophil-rich GPP as well as AGEP2728. We consistently observed intense IL-8 expression in the epidermis, especially in the pustular and parakeratotic areas. These findings reconfirmed that IL-8 is a key molecule for neutrophilic accumulation and pustular formation in GPP and AGEP. Furthermore, these findings suggest that both disorders might share a common pathogenesis for the development of pustular eruptions.
This study has some limitations. First, the biopsied specimens had different timing, i.e., each specimen has a different disease severity and course at the time of biopsy, which may influence protein expression. Furthermore, the proteins might become denatured during the paraffin-embedded procedure.
From this comparative immunohistochemical study, IL-36 and the IL-23/Th17 axis in GPP and AGEP were visualized and showed similar patterns except for IL-23 expression in the epidermis. In other words, it is not definite to differentiate two diseases by immunohistochemical methods. In conclusion, our results suggest that common pathomechanisms might exist in the development of GPP and AGEP. A future well-designed study with a large number of samples is needed to differentiate between GPP and AGEP.
Figures and Tables
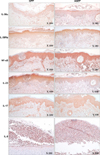 | Fig. 1The expression of cytokines and its receptor in GPP and AGEP. GPP: generalized pustular psoriasis, AGEP: acute generalized exanthematous pustulosis, IL: interleukin, IL-36Ra: IL-36 receptor antagonist, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells. |
Table 1
Clinical characteristics of patients with GPP and AGEP

Table 2
Comparison of immunohistochemical staining in GPP and AGEP

Values are presented as mean±standard deviation. GPP: generalized pustular psoriasis, AGEP: acute generalized exanthematous pustulosis, IL: interleukin, IL-36Ra: IL-36 receptor antagonist, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells. *Statistically significant difference between GPP and AGEP.
ACKNOWLEDGMENT
This study was supported by a grant from Ajou University Medical Center (M-2014-C0460-00051).
References
1. Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014; 53:676–684.

2. Varman KM, Namias N, Schulman CI, Pizano LR. Acute generalized pustular psoriasis, von Zumbusch type, treated in the burn unit. A review of clinical features and new therapeutics. Burns. 2014; 40:e35–e39.

3. Raison-Peyron N. "Cutaneous adverse drug reactions" are not always drug-induced. Eur J Dermatol. 2013; 23:439–442.

4. Abbas M, Holfeld K, Desjardins D, Zimmer J. Pustular psoriasis complicated with acute generalized exanthematous pustulosis. J Dermatol Case Rep. 2014; 8:42–45.

5. Choi MJ, Kim HS, Park HJ, Park CJ, Lee JD, Lee JY, et al. Clinicopathologic manifestations of 36 Korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature. Ann Dermatol. 2010; 22:163–169.

6. Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasis. J Cutan Pathol. 2010; 37:1220–1229.

7. Chang SL, Hu S, Hung SI, Huang YL, Hsiao WC, Chung WH. A comparison of Ki-67 antigen presentation in acute generalized exanthematous pustulosis and pustular psoriasis. Arch Dermatol Res. 2010; 302:525–529.

8. Schmid S, Kuechler PC, Britschgi M, Steiner UC, Yawalkar N, Limat A, et al. Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation. Am J Pathol. 2002; 161:2079–2086.
9. Duan H, Koga T, Kohda F, Hara H, Urabe K, Furue M. Interleukin-8-positive neutrophils in psoriasis. J Dermatol Sci. 2001; 26:119–124.

10. Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch Dermatol Res. 2012; 304:465–469.

11. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013; 34:174–181.

12. Sugiura K, Takemoto A, Yamaguchi M, Takahashi H, Shoda Y, Mitsuma T, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013; 133:2514–2521.

13. Kabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, Tokura Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J Eur Acad Dermatol Venereol. 2011; 25:485–488.

14. Song HS, Yun SJ, Park S, Lee ES. Gene mutation analysis in a korean patient with early-onset and recalcitrant generalized pustular psoriasis. Ann Dermatol. 2014; 26:424–425.

15. Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011; 89:432–437.

16. Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, Barker JN, Capon F, Creamer D, et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol. 2013; 133:1904–1907.

17. Kanazawa N, Nakamura T, Mikita N, Furukawa F. Novel IL36RN mutation in a Japanese case of early onset generalized pustular psoriasis. J Dermatol. 2013; 40:749–751.

18. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001; 28:113–119.

21. Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001; 167:1440–1446.

22. Teraki Y, Tanaka S, Hitomi K, Izaki S. A case of generalized psoriasiform and pustular eruption induced by infliximab: evidence for skin-homing Th17 in the pathogenesis. Br J Dermatol. 2010; 163:1347–1351.

23. Bissonnette R, Nigen S, Langley RG, Lynde CW, Tan J, Fuentes-Duculan J, et al. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J Eur Acad Dermatol Venereol. 2014; 28:1298–1305.

24. Ueda T, Abe M, Okiyama R, Oyama S, Satoh K, Aiba S, et al. Acute generalized exanthematous pustulosis due to allylisopropylacetylurea: role of IL-17-producing T cells. Eur J Dermatol. 2011; 21:140–141.

25. Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992; 258:1798–1801.

26. Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989; 243:1464–1466.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download