INTRODUCTION
Androgenetic alopecia (AGA) is an androgen-induced, progressive disorder, which is the most common type of alopecia after puberty. In AGA patients, hairs in affected area get thinner and shorter. A hairline recedes at the temples and the vertex gets bald.
AGA is often perceived as normal aging process, but many patients are suffering from AGA. DeMuro-Mercon et al.
1 revealed that 90% of Norwegian men aged 26~50 years self-reported having at least some hair loss. In this study, men who perceive themselves as having greater hair loss are more suffered from their hair loss. The prevalence of AGA in North East Asian (Japanese, Taiwanese, Chinese, and Korean) is lower than in Caucasians
2345. Patients visiting clinics are getting younger
6, and hair loss can significantly cause psychosocial manifestations, leading to economic on household health expenditure particularly in young patients
7.
Given the high prevalence, the treatment option for AGA is relatively limited though minoxidil and 5-alpha reductase inhibitors are widely used
8. Dutasteride 0.5 mg was recently added on the treatment options. Several studies proved that 0.5 mg of dutasteride improved hair growth and was relatively well tolerated for the treatment of AGA
91011. However, concerns on the safety and tolerability remain.
The objective of this study was to explore adverse events of dutasteride in Korean patients with AGA as per the requirement of Korea Ministiry of Food and Drug Safety.
MATERIALS AND METHODS
Subjects
Men 18 to 41 years of age diagnosed as AGA were eligible for this study if they will be treated with dutasteride 0.5 mg on the physician's medical judgement. Patients who previously treated with minoxidil or finasteride were eligible for this study but they must never have used dutasteride before for any reasons. Subjects who could not comply with the requirements of the protocol and follow the administration regimen were not enrolled. The institutional review boards approved the study protocol. The study was carried out from July 2009 to July 2013 at 21 centers in Korea (Ajou University Hospital, Asan Medical Center, Chonnam National University Hospital, Chung-Ang University Hospital, Chungbuk National University Hospital, Chungnam National University Hospital, Dankook University Hospital, Dong-A University Hospital, Inha University Hospital, Keimyung University Dongsan Medical Center, Konkuk University Medical Center, Korea University Ansan Hospital, Kyung Hee University Hospital at Gangdong, Kyungpook National University Hospital, Myongji Hospital, Samsung Medical Center, SMG-SNU Boramae Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Severance Hospital, Wonju Severance Christian Hospital). Approximately 900 subjects planned to be enrolled in order to provide 600 evaluable subjects for the primary analysis (according to Guideline for Korean New Drug Re-Examination
12) in consideration of an expected 30% drop out rate.
Study design
In the open-label, multi-center, observational study, dermatologists in Korea were asked to document on case report forms their observations for the contracted number of AGA subjects who were to receive dutasteride 0.5 mg daily on the physician's medical judgement.
Treatment
The subjects received dutasteride as monotherapy or as part of a combination therapy, based on the physician's medical judgement. In accordance with the prescribing information, dutasteride 0.5 mg once a day was prescribed. The treatment duration was not pre-specified.
Subject evaluation
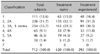
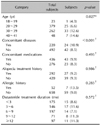
Each physician decided visit schedules based on their routine practices. At the initial consultation, demographic information (including age, height, and weight), medical history (including allergy history, family history, concomitant diseases, and AGA treatment history), modified Norwood-Hamilton classification, and concomitant medications were recorded. Physicians were guided to record any treatment-emergent adverse events during the follow up. At the last treatment visit, the effectiveness was additionally evaluated as "improved", "no change", "worsened" and "not assessed." The overall effectiveness assessment will depend on the physician's medical judgement.
Definitions
An adverse event (AE), which was coded based on World Health Organization Adverse Reactions Terminology 092, was defined as any untoward medical occurrence in a subject temporally associated with the use of a dutasteride, whether or not considered related to the medicinal product. Serious adverse event (SAE) is any untoward medical occurrence that at any dose; 1) results in death, 2) is life-threatening, 3) requires inpatient hospitalisation or prolongation of existing hospitalisation, 4) results in persistent or significant disability/incapacity, or 5) is a congenital anomaly/birth defect. An adverse drug reaction (ADR) was defined as all noxious and unintended responses related to dutasteride 0.5 mg. Physicians classified the relatedness into 6 category; "certain", "probable/likely", "possible", "unlikely", "conditional/unclassified," and "unassessable/unclassifiable." If the causality between dutasteride and AEs are considered "certain", "probable/likely", "possible" "conditional/unclassified," and "unassessable/unclassifiable," AEs were classified as ADRs.
After dutasteride treatment, the effectiveness was evaluated by treating physicians as "improved", "no change", "worsened", or "not evaluable". "Improved" is defined as an improvement or maintenance of the treating condition. Patients are classified as "improved" if the patient experienced hair growth or if the patient experienced comparable hair growth from prior treatment or if the patient experienced less hair loss. "No change" is defined as no improvement of the condition after treatment. Patients are classified as "no change" if the patient is on treatment and experiences the no improvements of hair loss compared to baseline or without any treatment. "Worsened" is defined as worsening of the condition after treatment. Patients are classified as "worsened" if the patient on treatment and experiences worsening of hair loss compared to baseline or without any treatment.
Statistical analysis
Continuous variables are expressed as the mean with one standard deviation and discrete variables are expressed as the frequency and rate. The primary objective was to evaluate the frequency and cases of AE after dutasteride administration. After all AEs were classified by preferred terms and system-organ classes, frequency and percent of each AE were estimated. Chi-square test or Fisher's exact test were used in order to check whether ADRs are associated with specific factors. Multivariate logistic regression was used to predict the factors affecting the frequency of ADR. The potential factors were initially evaluated separately, then all the factors with a p-value smaller than 0.2 were tested simultaneously in the multivariate logistic regression model. p-value less than 0.05 was considered statistically significant. Statistical calculation was carried out using SAS ver 9.2 (SAS Institute Inc., Cary, NC, USA).
DISCUSSION
Dihydrotestosterone (DHT) plays a key role in the pathogenesis of AGA. Although testosterone is a major circulating androgen, DHT, which is converted by 5-alpha reductase from testosterone, is more active in scalp hair follicles.
The role of type 2 5-alpha reductase in AGA has been supported by the men with a congenital deficiency of type 2 5-alpha reductase
13 and by the finasteride, a selective type 2 5-alpha reductase inhibitor
14. Type 1 5-alpha reductase is the major isoenzyme in sebaceous and sweat glands in skin
151617 and liver
17. However, the virilization at puberty of pseudohermaphrodites without type 2 5-alpha reductase associates with increasing type 1 5-alpha reductase expression in skin, suggesting that DHT can have paracrine effects
17. As a result, this suggests that the inhibition of both the type 1 and 2 5-alpha reductases may be needed to control DHT effectively.
Dutasteride inhibits both type 1 and 2 5-alpha reductases, eventually suppressing DHT in circulation and the target organ. In phase II trial, dutasteride from 0.05 mg to 2.5 mg suppressed serum and scalp DHT concentrations, which were inversely correlated with target area hair count, in a dose-related manner
11. Considering the fact that there was no dose-response relationship among finasteride groups
18, dutasteride's type 1 5-alpha reductase inhibition is likely to lead to the difference in dose-response of serum and scalp DHT inhibition. In the Olsen et al.'s report
11, dutasteride 0.5 mg suppressed DHT concentrations in serum by 92% and in scalp by 51%, while finasteride 5 mg in serum by 73% and in scalp by 41%. Recent study with bigger number of subjects showed the close dose response in hair count increase across dutasteride 0.02 mg, 0.1 mg and 0.5 mg
10 and added that dutasteride 0.1 mg was non-inferior to finasteride 1 mg and dutasteride 0.5 mg was superior to finasteride 1 mg.
In addition to the efficacy, it is believed that the AE is related to the DHT suppression. As a result, physicians perceive that the more potent DHT suppression by dutasteride, the more hair growth but the more AEs. Though this study does not have control group, the result on the safety in the real world can give some insights on safety information of dutasteride 0.5 mg in AGA.
In this observational study, decreased libido was reported in 9 subjects (1.3%), impotence in 7 (1.0%), sexual function abnormality in 4 (0.6%), and ejaculation disorder in 1 subject (0.1%). In dose ranging phase II trial, 9 men (13%) in the 2.5-mg dutasteride group complained of decreased libido, compared with 1 man (1%) in the 0.5-mg dutasteride group and 3 men (4%) in the finasteride 5 mg group
11. In Eun et al.'s report
9, sexually related AE was not different between dutasteride 0.5 mg group (4.1%) and placebo group (4.0%). Eun et al.
9 added that sexual dysfunction, which occurred for 3 of 73 (4.1%) in dutasteride group and 2 of 75 (2.7%) in placebo group and that both erectile dysfunction and ejaculation disorder was noted in one subject in placebo group. More recently, sexual AEs in all active groups (including dutasteride 0.02 mg, 0.1 mg, 0.5 mg and finasteride 1mg) comparing placebo group showed no dose response relationship in dutasteride doses
10. Gubelin Harcha et al.
10 also reported that decreased libido was reported by 9 subjects (4.9%) in dutasteride 0.5 mg group and by 12 subjects (6.7%) in finasteride 1 mg group.
The reports on the prognosis of the AEs in alopecia subjects are not found. In the 4-year follow-up of the phase III trials in benign prostate hyperplasia (BPH), the incidence of the sexual AEs was low and tended to decrease over time
19.
The reported sexually related AE in this observational study is relatively lower than other controlled studies. This difference might be from the age of study subjects. This observational study included male subjects from 18 to 41 years old per prescribing information while other studies enrolled up to 50 years old male.
Gynaecomastia is also one of the interesting AEs occurring after treatment with dutasteride. Gynaecomastia was reported in 2 subjects (0.3%) in this study. In phase II trial, the only subject to develop gynecomastia was in the placebo group and not in the dutasteride groups and finasteride groups
11. Gubelin Harcha et al.
10 reported that 1 subject (0.5%) experienced gynaecomastia in dutasteride 0.5 mg group and 1 subject (0.6%) in fiansteride 1 mg group. The frequency is about 1%~2% even in long term trials with thousands of older subjects for BPH
18. The reason for the lower gynaecomastia in trials for AGA may be from the shorter follow up period and the younger subjects.
Like other studies for AGA with dutasteride, this observational study showed no reports of prostate cancer, breast cancer, or cardiovascular AEs of special interest.
There are some limitations in this study in terms of assessment of the effectiveness and safety of the drug in a real practice with uncontrolled design. Firstly, though this study was designed to evaluate the safety and tolerability of the dutasteride, all the other treatment was allowed and more than half of subjects had other treatment as well. These additional medicines might have caused biases. Secondly, 380 subjects out of 712 in the safety population could not be assessed for effectiveness due to effectiveness assessment being not documented or effectiveness being not evaluable. The incomplete result data due to those subjects who were not assessed for effectiveness may have induced withdrawal bias. Thirdly, although 78.6% of the subjects in the analysis set were assessed to have improved in this study, there were no objective variables and determined time to assess effectiveness and therefore the status in those subjects who indicated improvement could not be confirmed. However, this study can provide the information on safety and effectiveness in a clinical practice environment.
In this study, we evaluated AEs of dutasteride in Korean patients with AGA in the real world. However, we relatively more focused on the ADR in this article because information on ADRs rather than AEs are more useful to the clinicians. Further evidences for safety and effectiveness of duatsteride in AGA are needed.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download