Abstract
Background
The differential diagnosis of psoriasis and seborrheic dermatitis can be difficult when both conditions are localized to the scalp without the involvement of other skin sites.
Objective
We aimed to evaluate the histopathological differences between psoriasis and seborrheic dermatitis on the scalp and identify favorable criteria for their differential diagnosis.
Methods
We evaluated 15 cases of psoriasis and 20 cases of seborrheic dermatitis of the scalp that had been clinicopathologically diagnosed. Skin biopsy sections stained with H&E were examined. Additional immunohistochemistry was performed, including Ki-67, keratin 10, caspase-5, and GLUT-1.
Results
On histopathological examination, mounds of parakeratosis with neutrophils, spongiform micropustules of Kogoj, and clubbed and evenly elongated rete ridges were significantly more frequently observed in psoriasis. Follicular plugging, shoulder parakeratosis and prominent lymphocytic exocytosis were significantly more common in seborrheic dermatitis. Moreover, significantly higher mitotic figures were observed in psoriatic lesions than in seborrheic dermatitis. Immunohistochemistry did not show any difference between psoriasis and seborrheic dermatitis.
Conclusion
Histopathological features favoring psoriasis include mounds of parakeratosis with neutrophils, spongiform micropustules of Kogoj, clubbed and evenly elongated rete ridges, and increased mitotic figures (≥6/high-powered field). Features indicating seborrheic dermatitis are follicular plugging, shoulder parakeratosis and prominent lymphocytic exocytosis. Immunohistochemistry was not helpful in differentiating psoriasis from seborrheic dermatitis.
Psoriasis and seborrheic dermatitis are relatively common inflammatory skin disorders that may present with erythematous scaly patches. Psoriasis usually presents with thick silver-white plaques on the scalp, trunk, and extremities, especially on the extensor surface of the extremities. Its diagnosis is facilitated by the presence of characteristic nail changes (e.g., nail pitting, subungual hyperkeratosis, onycholysis) or the Auspitz sign. Seborrheic dermatitis is characterized by sharply demarcated, yellow to red to brown, and greasy or bran-like scaling patches and plaques, most commonly on the scalp, nasolabial folds, ears, eyebrows, and presternal chest. However, when both conditions are localized on the scalp with no involvement of other skin sites, the differential diagnosis between seborrheic dermatitis and psoriasis can be difficult due to their similar clinicopathological features. Furthermore, the two conditions can occasionally co-exist. Therefore, we conducted a retrospective observational study to evaluate the histopathological differences between them and find favorable criteria for their histopathological differential diagnosis.
Study approval was granted by the Ajou University Hospital Institutional Review Board (IRB no. MED-KSP-12-376). We evaluated 15 cases of psoriasis and 20 cases of seborrheic dermatitis of the scalp that had been clinicopathologically diagnosed between March 2004 and July 2013 at our institute. The patients enrolled in the psoriasis group had clinically evident psoriatic plaques with silver-white scale on areas other than scalp that was histopathologically confirmed as psoriasis. When the patients accompanied skin lesions on eyebrows, ears, and other intertriginous areas, they were excluded from the psoriasis group. The enrolled patients in the seborrheic dermatitis group had greasy scaling patches on seborrheic areas other than scalp which was confirmed as seborrehic dermatitis on biopsy.
Skin biopsies were obtained from the enrolled patients. We examined H&E-stained sections of the skin biopsies. The slides were independently examined by three blinded observers with respect to the following histologic parameters: amount of plasma within the mounds of parakeratosis (scant or prominent), presence of mounds of parakeratosis with neutrophils, spongiform micropustules of Kogoj, follicular plugging, dilation of the infundibulum, shoulder parakeratosis, epidermal spongiosis (slight or prominent), lymphocytic exocytosis (mild or moderate), clubbed rete ridges, rete ridges of even lengths, thinning of the suprapapillary plate, edema of the papillary dermis, extravasated erythrocytes, tortuous blood vessels in the papillary dermis that nearly touch the epidermis, dermal inflammatory cell infiltration (mild, moderate, or dense), and the number of mitotic figures.
Immunohistochemistry was performed on the formal-in-fixed, paraffin-embedded sections. The antibody panel included Ki-67 (1:100 dilution; Thermo Fisher Scientific, Fremont, CA, USA), keratin 10 (1:100 dilution; Abcam, Cambridge, MA, USA), caspase-5 (1:100 dilution; Abcam), and GLUT-1 (1:200 dilution; Thermo Fisher Scientific). The expression of each antibody except GLUT-1 was calculated as the ratio of the stained area to the measured epidermal area. For the statistical analysis, an image analysis program (Image-Pro PLUS version 4.5 software; Media Cybernetics, Silver Spring, MD, USA) was used. GLUT-1 expression was evaluated with respect to intensity (mild, moderate, or strong), localization (basal, suprabasal, or both), and distribution (basal layer-dominant or diffuse). A basal layer-dominant pattern indicated particularly strong staining of the basal layer, whereas a diffuse pattern indicated diffuse staining of the entire epidermis.
A Mann-Whitney U test and Fisher's exact test were performed using IBM SPSS Statistics Desktop 20.0.0 software (IBM Co., Armonk, NY, USA). Values of p<0.05 were considered to be statistically significant. A receiver operating characteristic (ROC) curve was used to determine a cut-off value for the number of mitotic figures.
The 4 women and 11 men in the psoriasis group were aged 8~74 years (mean age, 39.9 years). The 8 women and 12 men in the seborrheic dermatitis group were 16~79 years (mean age, 47.2 years). There were no significant differences between the two groups with respect to age or sex (p>0.05).
The histological features of the two groups are summarized in Table 1. Clubbed and evenly elongated rete ridges were more common in the psoriasis group than in the seborrheic dermatitis group (p<0.05; Fig. 1A, B). Mounds of parakeratosis with neutrophils and spongiform micropustules of Kogoj were more frequently observed in the psoriasis group than in the seborrheic dermatitis group (p<0.05). Follicular plugging and shoulder parakeratosis was also significantly more common in the seborrheic dermatitis group than in the psoriasis group (Fig. 1C, D). Lymphocytic exocytosis was significantly more severe in the seborrheic dermatitis group: 9 cases (45%) of seborrheic dermatitis showed moderate lymphocytic exocytosis versus 1 case (6.7%) of psoriasis (p<0.05). Although there was no significant difference in the degree of epidermal spongiosis between the 2 groups, prominent spongiosis presented more frequently in seborrheic dermatitis: all of the psoriasis cases showed mild spongiosis only, whereas 21.1% of the seborrheic dermatitis cases presented marked spongiosis. Tortuous blood vessels nearly touching the epidermis were frequently observed in the psoriasis group; however, the difference was not statistically significant. There were no significant differences in the amount of plasma within parakeratosis, dilatation of the infundibulum, thinning of the suprapapillary plates, edema within the papillary dermis, extravasation of erythrocytes, or dermal inflammatory cell infiltration.
Significantly increased numbers of mitotic figures were observed in the psoriasis group compared to the seborrheic dermatitis group (mean±standard deviation [SD], 5.1±2.1 vs. 4.3±1.5; p<0.05). We determined the best cut-off value for differentiating between seborrheic dermatitis and psoriasis of the scalp based on the average number of mitotic figures per single high-powered field (HPF) using the ROC curve with a significant area of 0.632 (95% confidence interval, 0.439~0.824). The cut-off of ≥6 mitotic figures per single HPF generated 90.0% specificity and 33.3% sensitivity (Youden index, 0.233), which represents the mathematically optimum differential point between psoriasis and seborrheic dermatitis (Table 2).
The expressions of Ki-67, keratin 10, and caspase were compared using the ratio of the stained area to measured epidermal area and did not differ significantly between the psoriasis group and the seborrheic dermatitis group (Table 3). In the psoriasis group, GLUT-1 was expressed mildly in 1 case (6.7%), moderately in 12 cases (80.0%), and strongly in 2 cases (13.3%). In the seborrheic dermatitis group, GLUT-1 was expressed mildly in 3 cases (15.0%), moderately in 14 cases (70.0%), and strongly in 3 cases (15.0%). GLUT-1 was expressed in both the basal and suprabasal areas in all of the patients. GLUT-1 was stained in a diffuse pattern in 1 of the 15 cases of psoriatic lesions (6.7%) and in 6 of 20 cases of seborrheic dermatitis (30.0%; Fig. 2). There was no significant difference in GLUT-1 distribution between the groups (Table 4).
Making the clinical and histopathological differential diagnosis between seborrheic dermatitis and psoriasis vulgaris of the scalp can be difficult. Although both diseases may appear as scaly erythematous patches with itching1, psoriasis usually accompanies lesions on the extensor areas whereas seborrheic dermatitis involves other sebaceous gland-rich areas of the skin such as the eyebrows, ears, central chest and intertriginous areas. Also, scalp involvement frequently shows marked plaque formation, while seborrheic dermatitis is more likely to be ill-defined and show patchy appearance1. Clinical diagnosis of scalp psoriasis is often difficult because the lesions occasionally reside exclusively on the scalp and psoriatic plaque may not be eminent. Textbooks on dermatopathology propose several histopathologic features that may allow the differential diagnosis between these two conditions2345. However, there were no consistent criteria, and the difficulty in differentiation was mentioned in all of the textbooks. Therefore, we evaluated the histopathological and immunohistochemical findings of seborrheic dermatitis and psoriasis of the scalp and tried to identify favorable criteria for their differential diagnosis.
Histopathologically, Munro microabscesses and spongiform micropustules of Kogoj are of great value in the diagnosis of psoriasis vulgaris5. Although mild spongiosis may be seen in psoriasis, the presence of marked spongiosis suggests otherwise56. Accentuated spongiosis, mounds of parakeratosis with neutrophils predominantly at the follicular ostia, and more irregular acanthosis are histological features suggestive of seborrheic dermatitis35. On our histopathological examination, mounds of parakeratosis with neutrophils, spongiform micropustules of Kogoj, and clubbed and even lengths of rete ridges were observed significantly more frequently in the psoriasis group. Follicular plugging, shoulder parakeratosis and prominent lymphocytic exocytosis were significantly more common in seborrheic dermatitis than in psoriasis. These results were consistent with previous findings56. Several studies mentioned that more prominent tortuosity of blood vessels in the dermal papillae is observed in psoriasis, whereas in seborrheic dermatitis, there is abundant plasma in the mounds of parakeratosis35. However, there was no significant difference in blood vessel tortuosity in the dermal papillae or the amount of plasma within mounds of parakeratosis in our study. Although there was no significant difference in epidermal spongiosis in our study, prominent epidermal spongiosis was not observed in any psoriasis cases, but was seen in 21.1% of seborrheic dermatitis cases. Given the small number of cases in this study, marked spongiosis might be a favorable criterion for the diagnosis of seborrheic dermatitis.
In this study, significantly more mitotic figures were observed in psoriatic lesions than in seborrheic dermatitis lesions (mean±SD, 5.1±2.1 vs. 4.3±1.5, respectively; p<0.05). An increased number of mitotic figures in psoriasis versus seborrheic dermatitis is consistent with the findings reported by Ackerman3. Using the ROC curve, we obtained a cut-off value for the differential diagnosis of equal or greater than 6 mitotic figures in a single HPF favoring the diagnosis of psoriasis with 90.0% specificity and 33.3% sensitivity. However, the difference between the mean mitoses per HPF was 0.8, a difference of less than one cell in mitoses. Thus, the result not only suggests that the difference in mitotic figures might be helpful in the differential diagnosis of the two diseases, but also suggests that given the small difference, the number of mitosis should not be the sole indicator of either psoriasis or seborrheic dermatitis at present.
To date, information available for the immunohistochemical differential diagnosis between psoriasis and seborrheic dermatitis is limited. Several studies showed that Ki-67, an established marker of cell proliferation, is highly expressed in psoriatic lesions in contrast to low expression in the other inflammatory skin lesions in atopic dermatitis6 and lichen planus6 as well as in normal skin7. Keratin 10 expression was more substantially reduced in psoriasis than in atopic dermatitis in the previous study8. Salskov-Iversen et al.9 revealed that caspase-5 mRNA expression was significantly upregulated in psoriasis, but not in chronic atopic dermatitis and acute contact dermatitis, suggesting that caspase-5 mRNA upregulation may be an indicator of an increased inflammatory potential of psoriatic skin as a result of high inflammasome activity. Here we evaluated the expressions of Ki-67, keratin 10, and caspase-5, but found no significant differences between the two groups. In this study, mitotic rate evaluated on H&E-stained sections was statistically significant to differentiate the two conditions, but Ki-67 positivity was not. Although Ki-67 staining is known to be a good tool in confirming and comparing mitotic activity, it does not always correlate with mitotic activity, as shown in a previous study comparing Ki-67 labeling index and mitotic index10. Thus, in differentiating the two diseases, mitotic rate should be considered as a distinct factor from Ki-67 positivity and is likely to be more useful. Also, as the current study has relatively small case numbers in both two groups, a larger study seems necessary in order to confirm our results.
GLUT-1 is a uniporter protein that facilitates the transport of glucose across the plasma membranes in human cells. A rise in GLUT-1 level is suspected to contribute to the improvement of cellular glucose metabolism, and subsequently increase cell proliferation11. In an earlier study, GLUT-1 was not expressed in the epidermis of normal skin, whereas it was expressed in 76.6% of uninvolved and 86.7% of involved skin of psoriatic patients. The study also showed that GLUT-1 was expressed in basal and suprabasal locations of epidermis in 24 of 26 (80%) involved psoriatic lesions7. There are currently no studies on GLUT-1 expression in seborrheic dermatitis. In the present study, GLUT-1 expression was observed in all cases in both groups, while the expression of GLUT-1 was observed as basal and suprabasal patterns in all of the cases. Abdou et al.7 divided localization of GLUT-1 expression into diffuse and focal patterns. However, in the present study, all of the cases exhibited complete epidermal layer staining with GLUT-1, inconsistent with the previous study's findings. Hence, we divided localization into basal layer-dominant (staining of the basal layer) and diffuse. Most of the psoriasis cases (93.3%) and seborrheic dermatitis cases (70.0%) showed a basal layer-dominant pattern. The difference was not statistically significant. GLUT-1 expression intensity did not differ significantly between the two groups. As a result, immunohistochemistry was not useful in differentiating between psoriasis and seborrheic dermatitis. Additional studies with larger numbers of cases are necessary to confirm this finding.
In summary, histopathological features indicating psoriasis include mounds of parakeratosis with neutrophils, spongiform micropustules of Kogoj, clubbed and even lengths of rete ridges, and increased numbers of mitotic figures (≥6/HPF). Features favoring seborrheic dermatitis are follicular plugging, shoulder parakeratosis and prominent lymphocytic exocytosis. Immunohistochemistry including Ki-67, keratin 10, caspase-5, and GLUT-1 was not helpful in differentiating psoriasis from seborrheic dermatitis of the scalp.
Figures and Tables
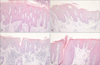 | Fig. 1Clubbed and even lengths of rete ridges were more common (A) in psoriasis than (B) in seborrheic dermatitis. Follicular plugging and shoulder parakeratosis were less common (C) in psoriasis than (D) in seborrheic dermatitis (H&E; A~D, ×200). |
 | Fig. 2Most cases showed basal layer-dominant GLUT-1 staining patterns in the psoriasis (93.3%; A) and seborrheic dermatitis (70.0%) groups. Although the proportion of diffuse pattern GLUT-1 distribution was higher in seborrheic dermatitis (B), the difference was not statistically significant (p=0.199; immunohistochemical GLUT-1 expression, ×400). |
Table 1
Histological features of 15 cases of psoriasis and 20 cases of seborrheic dermatitis

Table 2
Sensitivity, specificity, and Youden index values of cut-off points for the number of mitotic figures to differentiate between seborrheic dermatitis and psoriasis of the scalp

| Number of mitotic figures | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|
| ≥5.0 | 60.0 | 70.0 | 0.200 |
| ≥6.0 | 33.3 | 90.0 | 0.233 |
| ≥7.0 | 13.3 | 90.0 | 0.033 |
Table 3
Expression of Ki-67, keratin 10, and caspase-5 as the ratio of SA to measured EA

| Immunohistochemistry | Psoriasis (SA/EA) | Seborrheic dermatitis (SA/EA) | p-value |
|---|---|---|---|
| Ki-67 | 0.016±0.013 | 0.011±0.011 | 0.306 |
| Keratin 10 | 0.649±0.125 | 0.639±0.209 | 0.902 |
| Caspase-5 | 0.118±0.102 | 0.139±0.126 | 0.666 |
Table 4
Intensity, localization, distribution of expression of GLUT-1

References
1. Kim TW, Shim WH, Kim JM, Mun JH, Song M, Kim HS, et al. Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann Dermatol. 2014; 26:727–732.

2. Calonje E, Brenn T, Lazar AJ, McKee PH. McKee's pathology of the skin. 4th ed. Philadelphia: Elsevier/Saunders;2011. p. 180.
3. Ackerman AB. Differential diagnosis in dermatopathology II. Philadelphia: Lea & Febiger;1988. p. 2.
4. Braun-Falco O, Heilgemeir GP, Lincke-Plewig H. Histological differential diagnosis of psoriasis vulgaris and seborrheic eczema of the scalp. Hautarzt. 1979; 30:478–483.
5. Lever WF, Elder DE. Lever's histopathology of the skin. 10th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2009. p. 181.
6. Weedon D, Strutton G, Rubin AI. Weedon's skin pathology. 3rd ed. Edinburgh: Churchill Livingstone/Elsevier;2010. p. 80.
7. Abdou AG, Maraee AH, Eltahmoudy M, El-Aziz RA. Immunohistochemical expression of GLUT-1 and Ki-67 in chronic plaque psoriasis. Am J Dermatopathol. 2013; 35:731–737.

8. Bovenschen HJ, Seyger MM, Van de Kerkhof PC. Plaque psoriasis vs. atopic dermatitis and lichen planus: a comparison for lesional T-cell subsets, epidermal proliferation and differentiation. Br J Dermatol. 2005; 153:72–78.

9. Salskov-Iversen ML, Johansen C, Kragballe K, Iversen L. Caspase-5 expression is upregulated in lesional psoriatic skin. J Invest Dermatol. 2011; 131:670–676.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download