Abstract
Anaplastic large-cell lymphoma (ALCL) is a CD30-positive T-cell/null-cell lymphoma that is clinically classified into either primary cutaneous ALCL or systemic ALCL (S-ALCL) sub-types. Because 90% of childhood S-ALCL cases are anaplastic lymphoma kinase (ALK)-positive, there is a lack of data on ALK-negative S-ALCL cases among pediatric patients. Herein, we report a rare case of ALK-negative S-ALCL in a 9-year-old Korean boy who initially presented with itchy erythematous maculopapules and an erosive nodule on the trunk area. We emphasize the need of high index of suspicion of an underlying malignant disease in the presence of refractory eczematous lesions.
Anaplastic large-cell lymphoma (ALCL) is a CD30-positive T-cell lymphoma that accounts for 10%~15% of all pediatric non-Hodgkin's lymphoma (NHL)12. The World Health Organization (WHO) divides ALCL into two groups: 1) a spectrum of CD30-positive T-cell lymphoproliferative disorders that include lymphomatoid papulosis and primary cutaneous ALCL (C-ALCL) and 2) systemic ALCL (S-ALCL). S-ALCLs are further divided into anaplastic lymphoma kinase (ALK)-positive or ALK-negative subtypes. In pediatric patients, ALK-negative ALCL accounts for less than 5% of cases, for which there is a lack of data3.
Here, we present a rare case of CD30-positive ALK-negative S-ALCL in a child who initially presented with an eczematous skin condition.
A 9-year-old Korean boy presented with a two-month history of scattered erythematous maculopapules and an erosive nodule on his right upper abdomen (Fig. 1). At first, tiny itchy erythematous papular eruptions that did not respond to topical corticosteroid appeared, and then a nodular lesion developed. Although the nodular lesion was treated by incision and drainage along with oral antibiotics at another hospital, the patient did not improve and subsequently experienced a nighttime fever of 39℃. Skin biopsy from the erosive erythematous nodule showed pan-dermal infiltration of mixed inflammatory cells and pleomorphic large cells that had eccentric, horseshoe-shaped nuclei (Fig. 2A, B). Strong expression of CD30 in the membranes of these large cells was also observed (Fig. 2C). Other immunohistochemical staining of infiltrating cells indicated positive reaction to CD3, and CD4 with negative reaction to CD5, CD7, CD8, CD20, EMA, and ALK (Fig. 2D). Ki-67 was expressed over 70% of infiltrating tumor cells.
Laboratory findings revealed an elevated C-reactive protein level of 2.11 mg/dl (normal <0.3 mg/dl), while the other laboratory results were normal or negative. Whole-body positron emission tomography (PET) scanning showed multiple hypermetabolic lymph nodes in the mediastinum, neck, right axilla, right supraclavicular area, and both the pulmonary interlobular areas. Bone marrow biopsy confirmed involvement of CD30-positive atypical lymphocytes. Therefore, we diagnosed the patient with stage IV CD30-positive ALK-negative S-ALCL and started chemotherapy using the Berlin-Frankfurt-Münster regimen. After treatment, the scattered maculopapular lesions and the erosive nodular lesion all regressed, and, one year later, the patient has not experienced relapse (Fig. 3).
The diagnosis of ALCL is based upon the WHO classification with the feature of CD30 positive "hallmark cells". Hallmark cells are medium to large pleomorphic cells with eccentric nuclei that are usually horseshoe- or kidney-shaped4. ALCL is a rare disease in children accounting for about 15% of childhood NHLs and there are limited clinical data regarding S-ALCL in pediatric patients. A previous study of a pediatric Korean population showed that the incidence of childhood T-cell NHL, including S-ALCL cases, is higher than that in a similarly aged Western population (20.5% vs. 10.3%), which is consistent with previous findings of T-cell-type dominance among Korean patients56.
Among children, 18%~25% of S-ALCLs develop skin manifestations during the course of disease but S-ALCL presenting as skin lesion is very rare3. The clinical presentation of C-ALCL is characterized by the solitary or locoregional occurrence of reddish-brown nodules and tumors, which frequently ulcerate. Fifty percent of patients with primary C-ALCL present with solitary lesions and 25% present with generalized lesions7. It is often difficult to distinguish between primary C-ALCL and S-ALCL with cutaneous involvement, but making the correct diagnosis is imperative because treatment strategies differ. Physicians usually consider phenotype, clinical course, and morphology during differential diagnosis.
ALCL immunophenotypic characteristics of C-ALCL versus S-ALCL could be distinct but may also vary within the subtypes. Because most C-ALCLs are ALK-negative and most S-ALCLs are ALK-positive, ALK status has been thought to be the most helpful feature to differentiate between the two conditions78. However, there have been anecdotal case reports of ALK-positive C-ALCL cases and ALK-negative S-ALCL cases, such as in this case910. A previous report discussed ALK-negative ALCL cases that were positive for clusterin but negative for CD3 and CD8, with variable CCR4 expression. The cytotoxic proteins granzyme B, perforin, and T-cell intracytoplasmic antigen-1 are seen in more than 80% of ALK-positive S-ALCL cases and in 50% of ALK-negative S-ALCL cases11. Although in other ALK-negative S-ALCL cases, CD3 positivity, CD4 negativity, and CD8 positivity were observed, our case had different T-cell marker immunophenotypic features10. Because of this immunophenotypic variability, we considered C-ALCL in the differential diagnosis for a CD30-positive ALK-negative ALCL with cutaneous involvement. However, with multiple peripheral [18F] fluoro-2-deoxyglucose uptake detected on the PET-scan and involvement of the bone marrow with atypical lymphoid cells, we finally made a diagnosis of ALK-negative S-ALCL with skin involvement.
Treatment options for pediatric ALCL vary, but systemic polychemotherapy is indicated for S-ALCL, whereas a combination of surgical resection and radiation is the treatment of choice for C-ALCL. There is no consensus regarding a standard treatment for S-ALCL; some oncologists use short-pulse chemotherapy and others use prolonged semicontinuous leukemia-type chemotherapy or repeated pulse-chemotherapy3. Although S-ALCL is highly chemosensitive, with complete remission rates ranging from 66% to 100%, approximately 20 to 40% of patients will develop recurrent disease1213.
S-ALCL prognosis has been reported to correlate with ALK expression, which most frequently occurs as a result of a t(2;5)(p23q35) translocation, which fuses the ALK gene on 2p23 to the nucleophosmin (NPM) gene on 5q35, activating a unique NPM-ALK protein that acts like an oncogene. ALK expression is specific to ALCL, because it is absent in postnatal human tissue, except in rarely occurring brain cells. It is also absent from lymphoid neoplasms other than ALCL and ALK-negative large B-cell lymphomas4. ALK-positive S-ALCL often occurs in males who are younger than 30 years old and has a more favorable prognosis, while ALK-negative S-ALCL usually affects older patients (>60 years old) of both genders and has an unfavorable prognosis. However, different studies have reached various conclusions about the correlation of various prognostic factors with survival and the specific features that are associated with ALK-positive versus ALK-negative disease4121415. The lack of a systemic review of ALK-negative S-ALCL in pediatric patients may be a reflection of its rarity, making up ~10% of ALCL cases. Therefore, whether the excellent prognosis of ALK-positive S-ALCLs is due to younger age alone or is affected by presentation stage, clinical parameters, and ALK fusion proteins remains controversial.
A large European intergroup study on childhood ALCL showed that 10% of ALCLs are ALK-negative S-ALCL. B symptom, mediastinal involvement, skin lesions, visceral involvement, St. Jude stage 3~4, Ann Arbor stage 3~4, and elevated lactate dehydrogenase increased the risk of progression/relapse in univariate analysis. In multivariate analysis, three factors remained significant: mediastinal involvement, visceral involvement and skin lesions12. A Japanese study showed similar ALK expression and outcomes as the patients in the European reports16. However, a Chinese study found a higher percentage of ALK-negative S-ALCL in a pediatric patient group (15/46, 32.6%)15. In that study, S-ALCL patients who had complete remission after induction chemotherapy had a better prognosis, and clinical stage at the time of diagnosis was the only significant independent prognostic factor15. Our patient also achieved complete remission after induction chemotherapy and we have seen no evidence of recurrence thus far. Ethnic differences in the distribution or prognostic value of ALK expression in childhood ALCL cases are not well understood and should be studied further.
In this case, an initial skin lesion had an eczematous feature and another newly developed nodular lesion was thought to be an infectious disease such as abscess before taking a biopsy. Therefore, if the eczematous skin lesion persists in a waxing-waning pattern or has systemic symptoms, the attending physician should have a high level of suspicion of malignant disease and a skin biopsy should be performed. Moreover, classification of ALCL subtypes with clinical and immunotypical features is important for treatment and prognosis. Increasing knowledge of rare lymphoma subtypes may improve progress in treating this disease.
Figures and Tables
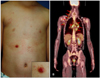 | Fig. 1(A) Scattered erythematous maculopapules and an erosive nodule on the trunk (inset: close-up view). (B) Arrows indicate multiple hypermetabolic [18F] fluoro-2-deoxyglucose uptake observed on positron emission tomography. |
 | Fig. 2(A) A skin biopsy specimen from the erosive nodule showed epidermal erosion and diffuse dermal cellular infiltration (H&E, ×12.5). (B) The infiltration consisted of large atypical cells with eccentric nuclei admixed with inflammatory cells (H&E, ×200). Immunohistochemical staining of the specimen showed positivity for (C) CD30 with a membranous staining pattern on large cells (×100), as well as negativity for (D) anaplastic lymphoma kinase (×100). |
References
1. Sandlund JT, Pui CH, Roberts WM, Santana VM, Morris SW, Berard CW, et al. Clinicopathological features and treatment outcomes of children with large cell lymphoma and t(2;5)(p23;q35). Blood. 1994; 84:2467–2471.

2. Wright D, McKeever P, Carter R. Childhood non-Hodgkin lymphomas in the United Kingdom: findings from the UK children's cancer study group. J Clin Pathol. 1997; 50:128–134.

3. Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013; 30:509–519.

4. Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of histopathologic, genetic and clinical features. Blood. 2000; 96:3681–3695.
5. Hwang IG, Yoo KH, Lee SH, Park YH, Lim TK, Lee SC, et al. Clinicopathologic features and treatment outcomes in malignant lymphoma of pediatric and young adult patients in Korea: comparison of korean all-ages group and Western younger age group. Clin Lymphoma Myeloma. 2007; 7:580–586.

6. Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005; 131:39–49.

7. Kocabaş E, Türel Ermertcan A, Akinci S, Temiz P, Gündüz K. Primary cutaneous CD30-positive anaplastic large cell lymphoma in a 16-year-old girl. Int J Dermatol. 2012; 51:1353–1358.

8. Kim HS, Sim SJ, Kim DC, Kim JS, Song KH, Kim KH. A case of ALK-negative systemic anaplastic large cell lymphoma. Ann Dermatol. 2004; 16:125–131.

9. Oschlies I, Lisfeld J, Lamant L, Nakazawa A, d'Amore ES, Hansson U, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica. 2013; 98:50–56.

10. Ju E, Adigun C, Dunphy C, Gold S, Morrell DS. Anaplastic large cell lymphoma: an unusual presentation in a 7-year-old girl. Pediatr Dermatol. 2012; 29:498–503.

11. ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Meijer CJ. ALK-negative systemic anaplastic large cell lymphoma: differential diagnostic and prognostic aspects--a review. J Pathol. 2003; 200:4–15.

12. Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008; 111:1560–1566.

13. Brugières L, Quartier P, Le Deley MC, Pacquement H, Perel Y, Bergeron C, et al. Relapses of childhood anaplastic large-cell lymphoma: treatment results in a series of 41 children--a report from the French Society of Pediatric Oncology. Ann Oncol. 2000; 11:53–58.

14. Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the international peripheral T-Cell lymphoma project. Blood. 2008; 111:5496–5504.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download