Abstract
Background
Chronic hand eczema (CHE) tends to be refractory to conventional therapy. Previous clinical trials have found that a 24-week treatment course of oral alitretinoin is effective and well tolerated for CHE treatment.
Objective
The aim of this study was to investigate the efficacy and safety of oral alitretinoin in Korean CHE patients.
Methods
A total of 27 patients with moderate to severe CHE took 30 mg of alitretinoin daily for 12 weeks. The primary efficacy parameter was the physician's global assessment (PGA). The response was defined as a patient being "clear" or "almost clear" of disease. The secondary efficacy parameters were the modified total lesion symptom score (mTLSS) and the patients' global assessment (PaGA). All adverse events and laboratory abnormalities were recorded during the treatment period.
Hand eczema (HE) is a common inflammatory skin disorder characterized by erythema, edema, papules, vesicles, scaling, fissures, hyperkeratosis, itching, and pain12. HE is often associated with irritant contact dermatitis, allergic contact dermatitis, or atopic dermatitis. However, the etiology can be multifactorial, and it is therefore difficult to clarify the specific cause(s)34. Mild HE is usually treated with use of emollients or application of topical corticosteroids, and patients are advised to avoid irritants5. However, HE develops into a chronic condition in a number of patients in spite of treatment, resulting in significant harmful effects to the patient's quality of life3456.
Alitretinoin is an endogenous physiological vitamin A derivative (retinoid) that has been developed for treatment of chronic HE (CHE)7. Several clinical trials have shown that taking alitretinoin is effective in treating severe CHE24589. Recently, alitretinoin has also been adopted in Korea for CHE treatment. This study was designed to evaluate the efficacy and safety of 12-week alitretinoin therapy for the treatment of CHE in Korean patients.
This was an open-label, prospective, non-comparative study. The study was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB No. 2013-09-008), and informed consent was obtained from all individuals included in the study.
Study participants included patients who visited the Department of Dermatology at Hanyang University Seoul Hospital in Korea between April 2014 and April 2015. Patients over 18 years of age who were diagnosed with moderate to severe CHE for a duration of at least three months and were refractory to topical corticosteroids were screened. The severity of CHE was defined using the physician's global assessment (PGA, see "Efficacy and Safety Assessments")5. Patients who had previously participated in an alitretinoin clinical trial and were unable to attend regular follow-up visits were excluded. Patients with all types of CHE including hyperkeratotic, vesicular (pompholyx), or fingertip eczema defined according to the German guidelines "Guideline on the Management of Hand Eczema"10 were included. A personal history of atopic dermatitis was obtained, and total serum immunoglobulin E concentrations were measured.
The main exclusion criteria were as follows: (1) any hypersensitivity to retinoids or vitamin A derivatives, (2) active atopic dermatitis or psoriasis requiring medical treatment, (3) allergic contact dermatitis of the hands where the patient is unable to avoid exposure to the allergen, and (4) active infection of the hands or other skin diseases likely to interfere with the conduct of the study. Other exclusion criteria were treatment with other investigational drugs within the previous two months, phototherapy, systemic steroids, treatment with retinoids or immunosuppressants within the previous four weeks, ingestion of drugs with potential for drug-drug interactions (i.e., systemic azoles, erythromycin, clarithromycin, or simvastatin) within the previous two weeks, and concomitant retinoids or vitamin supplements containing vitamin A. Also, those with alanine aminotransferase and/or aspartate aminotransferase values >2.5 times the upper limit of normal and fasting triglycerides and/or cholesterol >1.5 times the upper limit of normal were excluded. Women of reproductive age were prescribed alitretinoin only after negative urine pregnancy tests and contraceptive counseling. Contraception was required for one month after treatment cessation.
The treatment regimen consisted of 30 mg oral alitretinoin (Alitoc; Daewoong Co. Ltd., Seoul, Korea) once daily after meals for 12 weeks. If needed, a dose reduction from 30 mg to 10 mg or symptomatic management, including oral acetaminophen or antihistamines for a limited time period, was allowed in order to manage adverse events. Constant use of emollients was permitted, but concomitant topical and systemic corticosteroids, retinoids, phototherapy, or other systemic medications (immunosuppressants, tetracyclines, ketoconazole, itraconazole, erythromycin or clarithromycin, cyclosporine, and simvastatin) were prohibited.
Efficacy parameters were evaluated at screening and every week during the treatment, or at the last evaluation in the case of premature withdrawal.
Assessment of disease severity was done using PGA, the primary efficacy parameter. The secondary efficacy parameters were the modified total lesion symptom score (mTLSS) and the patients' global assessment (PaGA) of improvement, as utilized in previous studies2459. PGA classifies the severity of CHE into five categories (clear, almost clear, mild, moderate, and severe)5. A response was defined as a PGA assessment of either "clear" or "almost clear"5. The mTLSS was evaluated by the investigators as the sum of scores (0=absent, 1=mild, 2=moderate, and 3=severe) for each parameter (erythema, scaling, lichenification/hyperkeratosis, vesiculation, edema, fissures, and pruritus/pain)5. The PaGA was rated by the patients' subjective measures in improvement of disease as "clear or almost clear" (at least 90% clearing of disease signs and symptoms compared with baseline), "marked improvement" (75%~90% clearing), "moderate improvement" (50%~75% clearing), "mild improvement" (25%~50% clearing), or "no change or worsening"5.
The relapse rate and time to relapse were recorded among the responders. Relapse was defined as moderate to severe disease within 24 weeks of the end of treatment5.
Safety was evaluated at every visit by monitoring adverse events and participating in a physical examination. Laboratory tests (complete blood cell count, fasting blood chemistry, thyroid function test, and urine analysis) was performed at every 4 week.
Data from all available patients were analyzed descriptively. All efficacy analyses were based on the intention-to-treat population. A paired t-test was used to demonstrate the change in the mTLSS score. A correlation analysis was done via Kendall's τ correlation coefficient and the Spearman correlation coefficient using the statistics program IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
A total of 27 patients were enrolled in this study. Of them, 19 patients (70.3%) finished the protocol and 8 patients (29.6%) were withdrawn before completion. The primary reason for withdrawal was adverse events (n=4, 14.8%), followed by failure to return or patient refusal to complete the study due to the expense (n=3, 11.1%), and insufficient response (n=1, 3.7%).
Patient demographics and characteristics are shown in Table 1. The proportion of severe disease was approximately 70% (18/27), and the mean duration of disease was approximately 3.5 years. In total, 70.3% (19/27) of patients presented with hyperkeratotic hand eczema, and 10 patients (37.0%) had atopic dermatitis as an etiological factor.
Of the 27 patients, 12 patients (44.4%) responded to the alitretinoin therapy, with PGA ratings of "clear" or "almost clear" after the 12 weeks of treatment (Fig. 1, 2). According to the baseline severity (Fig. 3), patients with moderate CHE (66.7%, 6/9) showed more improvement than severe CHE (33.3%, 6/18). Of the patients with atopic diathesis, 50.0% (5/10) responded to alitretinoin therapy, while 41.1% (7/17) of those with other etiologic factors responded to alitretinoin treatment (Fig. 4). With regard to type of CHE (Fig. 5), the response rate was highest in hyperkeratotic eczema (47.4%, 9/19), followed by fingertip eczema (37.5%, 3/8), and vesicular eczema (30.8%, 4/13). In fingertip eczema, the responses were limited to "almost clear", unlike the other types of CHE.
During the 24-week follow-up observation period among the 12 responders, three patients relapsed (25.0%); the median time to relapse was 3.67 weeks (2 to 6 weeks).
The mean mTLSS score changed from 13.44 to 6.67 (p<0.001), representing a 50.4% of reduction at the end of the treatment period (Table 2). In addition, for the PaGA, 40.7% (11/27) of patients assessed their CHE as having a "clear" or "almost clear" status after alitretinoin therapy (Table 2).
The PGA score demonstrated good agreement with the mTLSS (0.61, Spearman coefficient) and PaGA (0.77, Kendall's τ coefficient) scores in correlation analyses. Also, there was a good agreement between the mTLSS and PaGA scores (0.72, Spearman coefficient).
Table 3 summarizes all the reported adverse events during the treatment period; they are the typical side effects of oral retinoids. In total, 51.9% of patients (14/27) experienced one or more adverse events. The most frequent adverse event was headache (40.7%, 11/27). Most of the adverse events occurred within the first two weeks of the study. In five patients (45.5%), the symptoms resolved spontaneously or after administration of acetaminophen. A dose reduction of 30 mg to 10 mg was done for two patients (18.2%) for one or two weeks; when the headache improved, they returned to taking 30 mg of alitretinoin. However, treatment had to be discontinued in four patients (36.4%); headache was the most common reason for withdrawal.
Commonly described mucocutaneous symptoms were pruritus (11.1%, 3/27) and dryness (11.1%, 3/27). Symptoms were controlled with application of emollients or by taking one or two weeks of oral anti-histamines. Gastrointestinal symptoms such as nausea (3.7%, 1/27) and vomiting (3.7%, 1/27), as well as dizziness (3.7%, 1/27) and facial flushing (3.7%, 1/27) occurred in a few patients, although not severely.
No acute illness or severe adverse events were noted, and there were no reported laboratory abnormalities such as elevation of hepatic enzymes or bilirubin levels, increased serum cholesterol and triglycerides, or decreased thyroid hormone and thyroid-stimulating hormones.
HE is quite common, with an annual prevalence of approximately 10% in the general population211. It includes a wide spectrum of disease severity, and it is estimated that 5~7% of patients with HE develop severe CHE, and 2%~4% of patients are refractory to topical treatment17. HE is significant due to the burden of disease and its substantial impact on quality of life7. It causes significant psychological distress as well as social and economic problems34. Agner et al.12 reported that quality of life is associated with HE disease severity.
A number of treatment options for HE have been tried, but they have often been both inadequate and difficult to manage110. Use of topical corticosteroids is the current standard treatment for the short-term control of HE13. However, it has limitations including incidence of rebound flare-ups and lack of efficacy in severely affected patients1014. Also, long-term usage can result in atrophic skin and epidermal barrier injury1014. Phototherapy in combination with topical therapy may improve CHE symptoms, but it is quite inconvenient and requires numerous visits1015. Systemic therapies including cyclosporine or retinoids are off-license treatment options considered for severe CHE that is unresponsive to potent topical steroids; however, there is limited clinical evidence of their efficacy and they are associated with possible long-term toxicities161718.
Alitretinoin, 9-cis retinoic acid, is a panagonist that binds to retinoid acid receptors and retinoid X receptors. It is thought to act by interfering with various steps in the inflammation processes of CHE7. It has recently been accepted as the only licensed systemic agent for treatment of CHE in patients unresponsive to potent topical corticosteroids7.
In a blinded, placebo-controlled study, 30 mg or 10 mg of once-daily oral alitretinoin for 24 weeks achieved response rates of 47.7% and 27.5%, respectively for the treatment of severe CHE5. It is also available nowadays in Korea and we conducted this preliminary trial to evaluate the efficacy of 30 mg of oral alitretinoin for 12 weeks in treating CHE.
Our study revealed that daily doses of 30 mg of oral alitretinoin for 12 weeks produced clinically significant responses in a high proportion of patients (44.4%) with moderate to severe CHE. This result is higher than that of previous study, which showed around 30% at week 12, and it is comparable to 47.7%, which is the response rate at week 24 in previous study5.
Among the seven patients who did not show improvement in their CHE after 12 weeks, four patients received an additional 4~12 weeks of alitretinoin treatment, after which two patients' CHE showed significant improvement (data not shown). All of the efficacy parameters (PGA, mTLSS, and PaGA) demonstrated clinical improvement of CHE and also showed high consistency among them, emphasizing the favorable outcome of alitretinoin therapy.
With regard to the severity of CHE at baseline, both moderate and severe CHE had a good response rate. However, the improvement in moderate disease was better. When evaluated by morphological type of CHE, a slightly better response rate was observed in hyperkeratotic disease compared to fingertip and vesicular disease. These results were also consistent with prior studies24.
Atopic skin diathesis is known to be an important endogenous factor in the etiology of CHE19. In particular, childhood atopic dermatitis is generally associated with a poor HE prognosis, including both severity and duration12. Therefore, it is noteworthy that alitretinoin showed a favorable response in patients with underlying atopic skin diathesis in our study.
Compared to previous trials, the relapse in our study patients was less frequent but tended to be more rapid520. This may be because our patients underwent follow-up assessment at shorter intervals. Adverse events included known typical retinoid class effects. They were mostly transient and spontaneously resolved within four weeks or were easily controlled with symptomatic management.
In assessing each of the side effects, headache was found to be the one most frequently described (40.7%); the prevalence was higher than prior studies, ranging from 7.5% to 27%2459. Side effects were usually managed with transient dose reduction or symptomatic treatment, but headache was the only adverse event that led to premature withdrawal. Other adverse events included mucocutaneous symptoms like pruritus and dryness, gastrointestinal symptoms, dizziness, and facial flushing, but they were only temporary. No serious adverse event or change in laboratory test was noted.
This study was limited by its non-comparative design with a relatively small number of patients, as it was a single-centered analysis. However, this study was the first trial to evaluate the efficacy and safety of alitretinoin administration for treatment of CHE in patients in East Asia. The results demonstrated that 12-week treatment of 30 mg of alitretinoin daily was effective in treating CHE. Our positive findings compared to previous studies may be explained by the fact that the patients who participated in this study included some cases of moderate CHE. However, the results of this study are significant because they suggest that a 24-week treatment course may not be necessary for alitretinoin therapy. In the future, larger, randomized trials with a greater variety of subjects would be useful to determine if there are racial differences and to confirm the findings of this study in a larger sample size.
Figures and Tables
Fig. 1
Clinical photographs in two patients with chronic hand eczem at baseline and after 12 weeks of alitretinoin treatment.
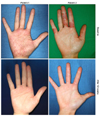
Fig. 2
Evaluation of the physician's global assessment (PGA) of chronic hand eczema after alitretinoin treatment. *Responders defined as 'clear' or 'almost clear' of disease.
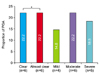
Fig. 3
Evaluation of physician's global assessment (PGA) according to severity of chronic hand eczema (CHE) at baseline. *Responders defined as 'clear' or 'almost clear' of disease.

Fig. 4
Evaluation of physician's global assessment (PGA) according to etiological factors. *Responders defined as 'clear' or 'almost clear' disease. †Irritant contact dermatitis, allergic contact dermatitis, or not identified.

Fig. 5
Evaluation of physician's global assessment (PGA) according to type of chronic hand eczema (CHE). *Responders defined as 'clear' or 'almost clear' disease. †Multiple nominations possible.

Table 1
Baseline patient demographics and characteristics

Table 2
Secondary efficacy parameters: mTLSS and PaGA

References
1. Diepgen TL, Agner T, Aberer W, Berth-Jones J, Cambazard F, Elsner P, et al. Management of chronic hand eczema. Contact Dermatitis. 2007; 57:203–210.

2. Diepgen TL, Pfarr E, Zimmermann T. Efficacy and tolerability of alitretinoin for chronic hand eczema under daily practice conditions: results of the TOCCATA open study comprising 680 patients. Acta Derm Venereol. 2012; 92:251–255.

3. Landow K. Hand dermatitis. The perennial scourge. Postgrad Med. 1998; 103:141–142. 145–148. 151–152.
4. Ruzicka T, Larsen FG, Galewicz D, Horváth A, Coenraads PJ, Thestrup-Pedersen K, et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch Dermatol. 2004; 140:1453–1459.
5. Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol. 2008; 158:808–817.

6. Yu M, Han TY, Lee JH, Son SJ. The quality of life and depressive mood among Korean patients with hand eczema. Ann Dermatol. 2012; 24:430–437.

7. Bissonnette R, Diepgen TL, Elsner P, English J, Graham-Brown R, Homey B, et al. Redefining treatment options in chronic hand eczema (CHE). J Eur Acad Dermatol Venereol. 2010; 24:Suppl 3. 1–20.

8. Bollag W, Ott F. Successful treatment of chronic hand eczema with oral 9-cis-retinoic acid. Dermatology. 1999; 199:308–312.

9. Dirschka T, Reich K, Bissonnette R, Maares J, Brown T, Diepgen TL. An open-label study assessing the safety and efficacy of alitretinoin in patients with severe chronic hand eczema unresponsive to topical corticosteroids. Clin Exp Dermatol. 2011; 36:149–154.

10. Diepgen TL, Elsner P, Schliemann S, Fartasch M, Köllner A, Skudlik C, et al. Deutsche Dermatologische Gesellschaft. Guideline on the management of hand eczema ICD-10 Code: L20. L23. L24. L25. L30. J Dtsch Dermatol Ges. 2009; 7:Suppl 3. S1–S16.

11. English J, Aldridge R, Gawkrodger DJ, Kownacki S, Statham B, White JM, et al. Consensus statement on the management of chronic hand eczema. Clin Exp Dermatol. 2009; 34:761–769.

12. Agner T, Andersen KE, Brandao FM, Bruynzeel DP, Bruze M, Frosch P, et al. EECDRG. Hand eczema severity and quality of life: a cross-sectional, multicentre study of hand eczema patients. Contact Dermatitis. 2008; 59:43–47.

13. Menné T, Johansen JD, Sommerlund M, Veien NK. Danish Contact Dermatitis Group. Hand eczema guidelines based on the Danish guidelines for the diagnosis and treatment of hand eczema. Contact Dermatitis. 2011; 65:3–12.

14. Meding B. Epidemiology of hand eczema in an industrial city. Acta Derm Venereol Suppl (Stockh). 1990; 153:1–43.
15. Halpern SM, Anstey AV, Dawe RS, Diffey BL, Farr PM, Ferguson J, et al. Guidelines for topical PUVA: a report of a workshop of the British photodermatology group. Br J Dermatol. 2000; 142:22–31.

16. Thestrup-Pedersen K, Andersen KE, Menné T, Veien NK. Treatment of hyperkeratotic dermatitis of the palms (eczema keratoticum) with oral acitretin. A single-blind placebo-controlled study. Acta Derm Venereol. 2001; 81:353–355.

17. Granlund H, Erkko P, Eriksson E, Reitamo S. Comparison of cyclosporine and topical betamethasone-17,21-dipropionate in the treatment of severe chronic hand eczema. Acta Derm Venereol. 1996; 76:371–376.
18. Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002; 147:324–330.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download