Abstract
Background
Moisturizers with anti-inflammatory or anti-itch activity should be developed for the safe and effective management of atopic dermatitis (AD).
Objective
This study evaluated the efficacy of a newly developed moisturizer, CSP0510 lotion (Twolines Inc., Korea), containing citric acid (CA) and trisodium phosphate (TSP) as active ingredients, in mild to moderate AD.
Methods and Results
CSP0510 lotion applied twice daily for 4 weeks to eczematous lesions improved objective and subjective (itch) symptoms of AD. The physician's global assessment (PGA) score for objective symptoms decreased from 2.5±0.6 before application to 1.3±0.5 after application in the CSP0510-treated group (n=42, p<0.001). Also, the PGA score decreased from 2.3±0.6 to 1.9±0.5 by vehicle-treated (without CA and TSP) control group (p=0.001), but there was no statistical difference between CSP0510-treated and vehicle-treated groups (p=0.089). The visual analogue scale score for itch decreased from 4.8±1.3 to 2.0±0.9 in the CSP0510-treated group (p<0.001), and from 4.6±1.1 to 3.5±0.9 in the control group (p=0.075), showing a statistical significance between two groups (p=0.002). Our results in humans were further supported by in vitro and animal experiments. In HaCaT cells treated with compound 48/80 (7.5 µg/ml), CA:TSP (1:1, vol:vol) synergistically suppressed the compound 48/80-induced upregulation of thymic stromal lymphopoietin, nerve grow factor, and calcitonin gene-related peptide. Application of CSP0510 to the dorsal skin of hairless mice for 3 weeks suppressed the oxazolone-induced allergic skin inflammation.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease that manifests with objective symptoms of eczematous skin lesions and subjective symptom of itch. It is a type of Th2-dominant immune response, especially in the acute stage of skin inflammation1. Cutaneous neurogenic inflammation (CNI), which is induced by the interaction between nervous system components and several target cells in the skin, also plays an important role in allergic skin inflammation and the itch sensation in AD2345. Neurotrophins (NTs), neuropeptides (NPs), chemokines, and cytokines have been proposed as candidate molecules for CNI3.
Thymic stromal lymphopoietin (TSLP) has been implicated in linking responses between our bodies and the environment in keratinocytes, thereby mediating Th2-type allergic inflammation by activating myeloid dendritic cells and mast cells67. In AD, TSLP is a crucial cytokine for inducing early skin inflammation, indicating that TSLP could be a good biomarker for evaluating Th2-type allergic inflammation in AD. As one of the major NTs in the skin, expression levels in the horny layer of nerve growth factor (NGF) are well correlated with AD severity58. Calcitonin gene-related peptide (CGRP), a type of NP, can increase interleukin (IL)-13 production in peripheral blood mononuclear cells from AD patients, suggesting that CGRP plays a role in modulating the Th2-type allergic inflammation in AD9. CGRP is a potent vasodilator that has been implicated in cutaneous thermal sensation or regulation of normal skin, as well as in CNI in AD1011. Hence, we selected TSLP, NGF, and CGRP as candidate biomarkers for evaluating the objective and subjective symptoms of AD.
CSP0510 lotion (Twolines Inc., Gwangju, Korea) was developed as a novel moisturizer for AD to improve skin inflammation and AD-related itch. CSP0510 lotion is a non-steroidal multi-component moisturizer in the form of an oil-in-water emulsion, in which citric acid (CA) and trisodium phosphate (TSP) were included as major ingredients. In relation to AD, CA was reported to have antibacterial activity against Staphylococcus aureus and TSP to have antifungal activity against Pityrosporum species12. We evaluated the efficacy of CSP0510 lotion for improving objective and subjective skin symptoms in AD. To further support the clinical studies, we tested the anti-inflammatory activity of CSP0510 lotion in the skin of oxazolone (OX)-treated hairless mice. OX acts as a hapten to induce allergic contact dermatitis, and repeated OX application induces skin inflammation of Th2 dominance, mimicking human AD13. We also tested the anti-inflammatory activity of CA, TSP, and CA:TSP (1:1, vol:vol) in the compound 48/80-treated HaCaT cells, a new, easy-to-use cell model of skin inflammation14.
CSP0510 lotion is a non-steroidal multi-component moisturizer, in which key ingredients are mixed together in the form of an oil-in-water emulsion. It is composed of the following ingredients: water, carbomer, glycerin, hydrogenated olefin polymer, cyclomethicone, butylene glycol, polysorbate 60, stearyl alcohol, stearic acid, polyethylen glycol (PEG)-100 stearate, glyceryl stearates, dimethicone, polyacrylamide/C13~14 isoparaffin/laureth-7, methylparaben, propylparaben, and disodium EDTA. The lotion was manufactured to contain 0.5% CA and 1.0% TSP in separate bottles to prevent chemical interaction between them during storage. AD patients were instructed to use the same volume of lotion from each bottle, and to mix them together in their palms just before application to the skin.
Patients with mild to moderate AD were enrolled, and were randomly allocated to a CSP0510-treated group (n=42) and a control group (n=42). AD was diagnosed using the criteria of Hanifin and Rajka15. The control group was supplied with lotion without CA and TSP. We performed a double-blinded controlled study to evaluate the efficacy of the lotion. All of the participants were instructed not to take oral antihistamines or steroids, or topical agents other than CSP0510 lotion or vehicle, during the experiments. This study was approved by the institutional review board of Chonnam National University Hospital (CNUH-2013-081) (Fig. 1). All of participants were provided a written informed consent form to be enrolled for this study. A written accent form was provided to one primary caregiving patent of enrolled children under 13-year-old. Patients were instructed to apply CSP0510 lotion with CA:TSP mixture (vol:vol=1:1, test group) or vehicle only (control group) to AD skin lesions twice per day for 4 weeks. The objective symptom of skin inflammation was scored using the Physician's Global Assessment (PGA) at the first visit (before application), second visit (after 2 weeks of application), and third visit (after 4 weeks of application). The degree of skin inflammation was scored as follows: 0=none; 1=minimal; 2=mild; 3=moderate; and 4=severe. Patients were also asked to score the relative intensity of their itch sensation between 0 and 10 using a visual analog scale (VAS).
Cell viability was assayed using the colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) kit (Chemicon International Inc., Billerica, MA, USA) according to the manufacturer's instructions.
We developed and applied a new, easy-to-use cell model to test allergic skin inflammation; compound 48/80 induces the upregulation of cyclooxygenase-2 via NF-κB activation14. Briefly, HaCaT cells were cultured to a sub-confluent state in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum and antibiotics, and were then treated with compound 48/80 in the presence or absence of CA (0.1 or 0.5 mM), TSP (0.1 or 0.5 mM), and CA:TSP (1:1, vol:vol) for 6 hours (TSLP) or 24 hours (NGF and CGRP). A polymerase chain reaction (PCR) pre-mixture kit (ELPIS, Daejeon, Korea) was used for reverse transcriptase-PCR. PCR reactions were performed with the following primers: human NGF (forward: 5'-catacaggcggaaccacact-3', reverse: 5'-gaattcgcccctgtggaaga-3'); human TSLP (forward: 5'-acaacttgtagggctggtgtt-3' ; reverse : 5'-tggcgaacatttctttggcg-3'); human CGRP (forward: 5'-ctgccacctgtgtgactcat-3' ; reverse : 5'-aaggctttggaacccacattg-3'), and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward: 5'-gtcttcaccaccatggagaaggc-3', reverse: 5'-cggaaggccatgccagtgagctt-3'). Annealing temperatures were as followed: 56℃ for human TSLP; 60℃ for human GAPDH; and 61℃ for human NGF and CGRP. Expression levels were normalized to the level of an endogenous control (GAPDH).
Hairless mice were purchased from Orient Bio (Daejeon, Korea). Animal experiments were approved by the Animal Care and Use Committee of Chonnam National University Hospital, Gwangju, Korea. The mice (female, 10 weeks old) were fed normal food and tap water, and raised in an individually ventilated cage rack system. As previously described, skin inflammation was induced by sensitization with 5% OX three times in the first week, followed by challenge with 0.5% OX three times per week (every other day) for the following 4 weeks13. From the third week, CSP0510 lotion was applied to the dorsal skin of mice three times a week for 3 weeks. For control mice, CSP0510l vehicle, which did not contain CA:TSP mixture, was applied with the same schedule as that for the test group. The degree of skin inflammation was scored as follows: 0=none; 1=minimal; 2=mild; 3=moderate; and 4=severe.
Frequency analysis and descriptive statistical analysis were used for analyzing the distribution of data, the averages and the standard deviations, etc. Wilcoxon signed rank test was performed to compare before and after the studies, and Mann-Whitney U test was used for comparing groups at each time. SPSS ver. 22.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data with p-values of <0.05 to be the statistically significant.
The mean age of patients was 17.6±2.5 years in the test group (n=42, male:female=1.2:1) and 18.5±3.6 years in the control group (n=42, male:female=1.1:1). Mean eczema area and severity index (EASI) score was 12.4±4.9 in the test group and 11.8±3.8 in the test group (no significant difference between the two groups). Objective symptom of skin inflammation was evaluated at each visit by the PGA. In the test group (n=42), mean PGA score decreased from 2.5±0.6 at the start (first visit) to 1.3±0.5 after 4 weeks of application (third visit) (p<0.001). In the control group (n=42), mean PGA score was 2.3±0.6 at the start and 1.9±0.5 after 4 weeks of application (p=0.001). The PGA score of tested group was lower than that of control group, but there was no statistical significance in PGA scores between two groups after four weeks of application (p=0.089, Fig. 2A). The mean VAS score in the test group decreased from 4.8±1.3 at the start (first visit) to 2.0±0.9 after 4 weeks of application (third visit) (p<0.001, Fig. 2B). In contrast, the mean VAS score in the control group decreased from 4.6±1.1 at the start to 3.5±0.9 after 4 weeks of application (p=0.075). The difference between the two groups after four weeks of application was statistically significant (p=0.002). Onset and duration of the anti-itch activity was 18.2±5.5 minutes and 205±9.5 minutes, respectively. After CSP0510 application, 85.7% (36/42) of the CSP0510-treated group responded "Yes" to our question "Would you recommend CSP0510 lotion to other AD patients?"
The 26.2% (11/42) of the test group complained of a transient burning sensation, compared to 5.5% (4/42) of the control group (p=0.001). However, all of participants did not stop using the lotion due to major adverse reactions.
As described before, compound 48/80-treated HaCaT cells were used as a cell model for skin inflammation14. In our preliminary experiments for cell viability, up to 12.8 µg/ml of compound 48/80 (A), 2.0 mM of TSP (B), and 5.0 mM of CA (C) were optimal, non-toxic concentrations for use in HeCaT cells (Fig. 3).
In this study, HaCaT cells were treated with 0.1~0.5 mM of TSA or CA. From our results that TSLP, NGF, and CGRP were up-regulated dose-dependently by 1~10 µg/ml of compound 48/80, the following experiments were performed with a concentration of 7.5 µg/ml of compound 48/80 in treating HaCaT cells (Fig. 4).
In compound 48/80-treated HaCaT cells, the compound 48/80-induced expression levels of tested biomarkers were suppressed by TSP (01 or 0.5 mM) or CA (0.1 or 0.5 mM) treatment. There was a statistical significance between compound 48/80-treated sample (control, 2nd lane) and compound 48/80, TSP-treated (3rd, 4th lanes) or compound 48/80, CA-treated (5th, 6th lanes) samples (p<0.001) (Fig. 5). Interesting, there was a synergistic effect by combining CA and TSP (1:1, vol:vol), showing a significant difference between CA:TSP-treated samples (7th, 8th lanes) and TSP- or CA-treated samples as a single agent (p<0.001). However, there was no dose-dependency between 0.1 mM- and 0.5 mM-treated samples in the suppressive activities of TSP or CA.
In the dorsal skin of OX-treated hairless mice, the PGA score increased from 0 (before OX application) to 3.6±1.35 after 5 weeks of OX application (n=5). The PGA score in OX-treated, CSP0510-treated mice (n=5) after 3 weeks of application was 2.1±0.85. The corresponding PGA score in OX-treated, vehicle-treated mice (n=5) was 2.8±1.20. The difference between the CSP0510 and control groups was statistically significant (p=0.002) (Fig. 6).
CSP0510 lotion was developed as a moisturizer containing two major ingredients of CA and TSP. Based on this study, we believe that CSP0510 lotion might be a novel moisturizer with therapeutic potential to relieve itch and skin inflammation in mild to moderate AD patients. Among anti-inflammatory and anti-itch activity of the lotion, CS0510 is revealed to be more satisfactory in anti-itch activity. Our result that the PGA score also decreased in the control group suggests that vehicle only can exert anti-inflammatory activity due to the action of ingredients in the lotion, but CA:TSP mixture has additive effect to enhance anti-inflammatory activity of lotion.
CA and TSP were reported to have antimicrobial activity against S. aureus and Pityrosporum species12. In the present study, we found that CA and TSP have a novel function of anti-itch and anti-inflammation in the skin, especially in AD patients. CA is found in various fruits, especially oranges, and is also found in the human body16. TSP is extensively used in formulations for a variety of soaps and detergents. It is used as cleaning agent, lubricant, food additive, stain remover, and degreaser17. CA and TSP were known to be safe for our bodies, including the skin. Originally, CA and TSP were expected to have anti-AD activity via their anti-microbial activities, but this study demonstrates that CA:TSP mixture synergistically exerts anti-inflammatory activity against Th2-type allergic inflammation in the skin. Consistently, CA was reported to have anti-allergic inflammatory activity in the skin of OX-treated hairless mice18. In their experiments, CA containing cream (pH 2.8) had anti-AD activity to decrease transepidermal water loss (TEWL) and to increase skin hydration in OX-induced skin inflammation. Lactic acid was also reported to have a similar activity to reduce skin inflammation and itch sensation in AD and non-AD patients with dry itchy skin1920. In together, the topical agents containing weak acids are expected to have anti-inflammatory and anti-itch activity in AD patients. At the same time, moisturizers containing acidic components might to have a potential to irritate the skin including burning sensation in sensitive AD patients21. Further studies should be focused to detect the ideal formulation of acids-containing moisturizers, which can exert strong anti-inflammatory and anti-itch activity without skin irritation.
Studies have demonstrated that CNI is a key mechanism for skin inflammation and itch in the pathogenesis of AD2345. In this study, we found that CA or TSP had inhibitory activity against compound 48/80-induced TSLP, NGF, and CGRP expression in HaCaT cells. Furthermore, there is a synergistic effect of CA and TSP by combining together in their suppressive activities against CNI. Anti-inflammatory activity of CA:TSP mixture was also identified in OX-induced skin inflammation in hairless mice and skin inflammation in AD patients in vivo.
Moisturizers are evolving as cosmeceuticals, which have more sophisticated functions, including therapeutic potential as drugs. Common ingredients of moisturizers with potential biological activities are α-hydroxy acids (AHAs), β-hydroxy acids (BHAs), retinoic acid, vitamins, and antioxidants22. As functional ingredients of moisturizers, AHAs have been widely used to improve dry skin as humectants, and also for hyperkeratotic skin as keratolytic agents23. Lactic acid, a monocarboxylic acid AHA, has been widely used to relieve itch as an ingredient of moisturizers for AD patients19. In the present study, we found that CA, a tricarboxylic acid AHA, also have a potential to inhibit CNI. Considering that itch relief might be an important goal of AD treatment, development of topical anti-itch agents with long-term safety such as functional moisturizers is of significant importance.
Taken together, our results indicate that AHAs including CA are important ingredients of moisturizers that improve subjective and objective symptoms of AD. In a preliminary study to compare efficacy of two moisturizers, CSP0510 lotion was found to be superior to Atopiclair (Sinclair Phrama Sr1, Milan, Italy); 73.1% of patients were satisfied with CSP0510 and 26.9% were satisfied with Atopiclair (n=26, unpublished data). Further multi-center, randomized, double-blinded studies of CSP0510 lotion are warranted to confirm our results.
Figures and Tables
 | Fig. 1The Consolidated Standards of Reporting Trials (CONSORT)-type flow chart of study population. AD: atopic dermatitis, CSP0510 lotion: Twolines Inc., Gwangju, Korea. |
 | Fig. 2Efficacy of CSP0510 lotion (Twolines Inc., Gwangju, Korea) for treating objective and subjective symptoms in atopic dermatitis (AD) patients. AD patients were randomized into test (n=42) and control (n=42) groups, and skin inflammation (an objective symptom) and itch (a subjective symptom) were scored using the physician's global assessment (PGA) and visual analog scale (VAS), respectively, at the first visit (before application), second visit (after 2 weeks of application), and third visit (after 4 weeks of application). (A) Time-course changes of PGA score in the CSP0510- and vehicle-treated (control) groups. (B) Time-course changes of VAS score in the CSP0510- and vehicle-treated (control) groups. Data represent the mean±standard deviation. NS: not significant. |
 | Fig. 3MTT assay for cell viability. The MTT assay was conducted in order to assess the cytotoxic activity of (A) compound 48/80 (0.1~100 µg/ml), (B) trisodium phosphate (TSP) (0.25~5.0 mM), and (C) citric acid (CA) (0.25~5.0 mM), respectively. |
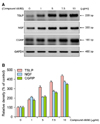 | Fig. 4Determination of the optimal dose of compound 48/80 in studying biomarkers for cutaneous neurogenic inflammation (CNI). (A) Reverse transcriptase- polymerase chain reaction (RT-PCR) experiments for thymic stromal lymphopoietin (TSLP), nerve growth factor (NGF), and calcitonin gene-related peptide (CGRP), biomarkers for CNI, were performed as described in the MATERIALS AND METHODS. HaCaT cells were treated with different doses of compound 48/80 (1~10 µg/ml) to find out the optimal dose of compound 48/80 in following experiments. (B) Densitometric analysis on the relative density of tested biomarkers are depicted. This picture represents three repetitive experiments of RT-PCR. |
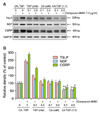 | Fig. 5Effect of citric acid (CA) and trisodium phosphate (TSP) on expression levels of thymic stromal lymphopoietin (TSLP), nerve growth factor (NGF), and calcitonin gene-related peptide (CGRP) in compound 48/80-treated HaCaT cells. (A) HaCaT cells were treated with TSP or CA or CA:TSP (1:1, vol:vol), and cells were harvested at designated time-points after treatment for reverse transcriptase- polymerase chain reaction (RT-PCR) experiments. Expression levels of TSLP were checked at 6 hours after compound 48/80 treatment, and those of NGF and CGRP were checked at 24 hours after compound 48/80 treatment. First lane represents the negative control samples, which were not treated with compound 48/80 and CA or TSP. Second land represents the positive control samples, which were treated only with compound 48/80 (7.5 µg/ml). (B) Data represent the mean±standard deviation of three independent experiments after densitometric analysis for positive bands of (A). *p<0.01 between the compound 48/80, TSP-treated and the compound 48/80, CA:TSP-treated samples. *p<0.01 between the compound 48/80, CA-treated and the compound 48/80, CA:TSP-treated samples. |
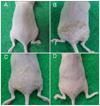 | Fig. 6Effect of CSP0510 lotion (Twolines Inc., Gwangju, Korea) on oxazolone-induced skin inflammation in hairless mice. (A) Nontreated mouse, (B) oxazolone (OX)-treated mouse, (C) OX-treated, vehicle-applied mouse (control) and (D) OX-treated, CSP0510-applied mouse. The results shown are representative of five mice in each group. |
ACKNOWLEDGMENT
This study was supported by a grant of Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 11079-21).
References
1. Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011; 127:1420–1432.

2. Ostlere LS, Cowen T, Rustin MH. Neuropeptides in the skin of patients with atopic dermatitis. Clin Exp Dermatol. 1995; 20:462–467.

3. Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998; 7:81–96.

4. Wang IJ, Hsieh WS, Guo YL, Jee SH, Hsieh CJ, Hwang YH, et al. Neuro-mediators as predictors of paediatric atopic dermatitis. Clin Exp Allergy. 2008; 38:1302–1308.

5. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009; 53:48–54.

6. Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009; 39:798–806.

7. Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012; 61:3–17.

8. Ikezawa Z, Komori J, Ikezawa Y, Inoue Y, Kirino M, Katsuyama M, et al. A role of staphyococcus aureus, Interleukin-18, nerve growth factor and semaphorin 3A, an Axon guidance molecule, in pathogenesis and treatment of atopic dermatitis. Allergy Asthma Immunol Res. 2010; 2:235–246.

9. Antúnez C, Torres MJ, López S, Rodriguez-Pena R, Blanca M, Mayorga C, et al. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br J Dermatol. 2009; 161:547–553.

10. Park TJ, Comer C, Carol A, Lu Y, Hong HS, Rice FL. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol. 2003; 465:104–120.

11. Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol. 2008; 22:223–228.

12. Jung CH, Cho HH, Choi GJ, Kang SY, Yang NW. The anti-sticking effect of mixture of trisodium phosphate and citric acid on oral streptococcus species. Korean J Microbiol. 2008; 44:289–292.
13. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008; 128:79–86.

14. Choi DI, Choi JY, Kim YJ, Lee JB, Kim SO, Shin HT, et al. Ethanol extract of peanut sprout exhibits a potent anti-inflammatory activity in both an oxazolone-induced contact dermatitis mouse model and compound 48/80-Treated HaCaT Cells. Ann Dermatol. 2015; 27:142–151.

15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980; 92:Suppl. S44–S47.
16. Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008; 22:567–570.

17. Xiong H, Li Y, Slavik MF, Walker JT. Spraying chicken skin with selected chemicals to reduce attached Salmonella typhimurium. J Food Prot. 1998; 61:272–275.

18. Lee HJ, Yoon NY, Lee NR, Jung M, Kim DH, Choi EH. Topical acidic cream prevents the development of atopic dermatitis- and asthma-like lesions in murine model. Exp Dermatol. 2014; 23:736–741.

19. Grove G, Zerweck C. An evaluation of the moisturizing and anti-itch effects of a lactic acid and pramoxine hydrochloride cream. Cutis. 2004; 73:135–139.
20. Vilaplana J, Coll J, Trullás C, Azón A, Pelejero C. Clinical and non-invasive evaluation of 12% ammonium lactate emulsion for the treatment of dry skin in atopic and non-atopic subjects. Acta Derm Venereol. 1992; 72:28–33.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download