Abstract
Background
Although the incidence of non-melanoma skin cancer is increasing, there are no effective practical preventive measures other than avoiding sun exposure.
Objective
To elucidate the protective effect of topical application of biologically active vitamin D3 (calcitriol) on skin cancer development caused by exposure to ultraviolet (UV).
Methods
Groups of hairless mice were topically treated with either calcitriol or vehicle immediately after exposure to UVB and UVA three times weekly for the initial 20 weeks, and without UV exposure in the following 6 weeks. Tumor number was counted and biopsies were done for histopathologic analysis. The changes of cyclobutane pyrimidine dimer (CPD) were evaluated 1 hour and 11 hours after short term of UV exposure and application of calcitriol. For safety evaluation, blood test and body weights were evaluated at 23rd and 25th week.
Results
Total tumor count and number of tumors less than 3 mm in size tended to be fewer in calcitriol group, and tumors more than 3 mm in size showed significantly lower tumor formation rate in calcitriol group. Single application of calcitriol reduced CPD at 1 hour and 11 hours after UV exposure. Histopathologic analysis showed tumors with lower grade malignancy in calcitriol group which suggested a delay in tumor progression. However, serum levels of calcium and phosphate in calcitriol group were above normal range, and weight loss was found.
Calcitriol [1α,25-dihydroxy-vitamin D3, 1,25(OH)2D] is a hormonally active vitamin D3 metabolite which has been widely used for treatment of psoriasis. Recent evidence suggests that calcitriol induces a set of local responses that may give rise to beneficial effect when it is produced in the skin123. These responses include the inhibition of proliferation, the acceleration of differentiation, an anti-inflammatory effect, the expression of antimicrobial peptides, an immunomodulatory effect, and an anti-cancer effect12345. Calcitriol is therefore known to be important for the maintenance of epidermal homeostasis. Furthermore, since the potential anti-cancer activity of calcitriol has been suggested in 19796, numerous basic and epidemiologic researches to support the hypothesis and assure the action mechanism have been conducted78. On the other hand, controversial studies exist indicating no prominent effect of topical vitamin D3 analogue (calcipotriol) on photocarcinogenesis910.
However, these previous novel studies dealt with the basic cellular mechanisms of vitamin D system, not the effect on actual in vivo cancer or its protective effects. There is little data about the role of topical active vitamin D3 in preventing the development of skin tumor in an animal photocarcinogenesis model. Therefore, we have hypothesized that topically applied active vitamin D3, calcitriol, may have a partial role in protecting against ultraviolet (UV)-induced photocarcinogenesis, and designed the study by using murine model of photocarcinogenesis that is similar to human photocarcinogenesis.
In a murine carcinogenic model, tumor formation and progression occurs sequentially in the following order: benign papilloma, dysplastic papilloma, keratoacanthoma, squamous cell carcinoma (SCC), and spindle cell carcinoma1112. Generally a size of tumor increases along with the sequence. It is different from human photocarcinogenesis, which initially shows a form of actinic keratosis. Though the experiment in this study was based on an animal model rather than a human model, it is so far a well-established model which may help explain the mechanism of photocarcinogenesis and its protection. Herein, we investigated the chemopreventive activity of topically applied vitamin D3 against tumor development in vivo in an SKH:hr-1 hairless mice skin model to elucidate whether the occurrence of skin cancer caused by exposure to UV light can be attenuated by topical application of biologically active vitamin D3. Furthermore, we aimed to reveal the anti-cancer mechanism of vitamin D3, and ultimately obtain safe and practical method for skin cancer prevention.
Female hairless mice (SKH:hr-1, 6~7 weeks old) were purchased from a specific pathogen-free colony at Oriental Inc. (Seoul, Korea) and was allowed for 1 week of quarantine and acclimatization. Mice were kept under conditions of controlled humidity (40%) and temperature (22±2℃). All animal experiments were conducted in accordance with accepted standards of humane animal care, under protocols approved by the Local Animal Research Committee at Yonsei University Wonju College of Medicine. The animals were housed five per polycarbonate cage, and were given tap water and commercial rodent chow (Samyang Feed Co., Korea) ad libitum.
A mice photocarcinogenesis model was used as previously described13. A total of 35 mice were divided into three groups, 15 in calcitriol group with topical application of calcitriol after UV-irradiation, 15 in vehicle group with topical application of vehicle with ointment base after UV-irradiation, and 5 in control group with sham light-irradiation without any topical application. The UV apparatus consisted of three UVB fluorescent lamps (G20T10E, 20 W; Sankyo Denki, Hirakuka, Japan) and five UVA lamps (F20T10, 20 W; Sankyo Denki). Irradiance was set up with 0.3 mW per cm2 for UVB and 4.5 mW per cm2 for UVA at a distance of 40 cm as measured by UV-Meter® (Waldmann, Villingen-Schwenningen, Germany). All mice were simultaneously exposed to 60 mJ/cm2 of UVB and 1.8 J/cm2 of UVA three times a week for 20 weeks. Control group was exposed to ordinary fluorescent lamp (sham light) (DULUX-L®; Oslam Korea, Ansan, Korea) for the same exposure time as other groups. Calcitriol ointment (calcitriol 3 µg/g, petrolatum base; Silkis®, Galderma, UK) or vehicle (petrolatum) was applied evenly to the back skin with 0.1 g per mouse 10 minutes after UV irradiation. UV treatment was stopped at 20th week, and for the following 6 weeks only the topicals were applied three times a week until 26th week.
Skin lesions were recorded as tumors if they were circular, erythematous, raised, greater than 1 mm in diameter, and persisted for two or more weeks. The total number of tumor per mouse was counted every two weeks over the 26 week period of the experiment. Based on the cut-off size of 3 mm, the number of tumors of each size (1~3 mm and ≥3 mm) was also assessed.
Nine of the largest tumors in each group were taken immediately after the final tumor evaluation at 26th week and fixed in 4% buffered formaldehyde. In order to evaluate the aggressiveness of tumor, a blinded dermatopathologist analyzed the type of tumor and its grade in six categories: 1, benign papilloma; 2, dysplastic papilloma; 3, keratoacanthoma; 4, well-differentiated SCC; 5, moderately-differentiated SCC; 6, poorly-differentiated SCC.
To validate the effect of topical calcitriol on DNA repair, two SKH:hr-1 hairless mice in each group were irradiated with 60 mJ/cm2 of UVB and 1.8 J/cm2 of UVA daily for two consecutive days, and either topical calcitriol or vehicle was applied 10 minutes after each irradiation. One and eleven hours after the last irradiation, skin samples were taken from the central back of mice, and embedded in optimal cutting temperature compound in cryomolds.
Immunofluorescence detection of cyclobutane pyrimidine dimer (CPD) in skin was done by using a monoclonal antibody TDM-2 (Cosmo Bio Co. LTD., Tokyo, Japan) and an enzyme-labeled secondary antibody Alexa Fluor 488 goat anti-mouse IgG (Life Technologies, Carlsbad, CA, USA). Before staining, slides were warmed at room temperature for 10 minutes and fixed in 0.07 M NaOH in 70% ethanol for 4 minutes, then it was washed 3 times for 5 minutes in phosphate buffered saline. Samples were incubated and blocked for 15 minutes with protein block (DAKO, Carpinteria, CA, USA). Primary antibody was incubated overnight at 4℃. After several washes in PBS, they were incubated for 1 hour with a secondary antibody at room temperature. After being washed 3 times for 5 minutes in PBS, it was counterstained with propidium iodide for 2 minutes. The completed slides were examined under a Leica TCS SP5 Confocal Microscope (Leica Microsystems, Tokyo, Japan).
Body weight was checked at 23rd and 25th week of experiment. To assess a comprehensive metabolic panel, blood from all mice in each group were sampled from ophthalmic vein for measurement of complete blood count (CBC) and serum chemistry profile at the end of the experiment. Serum chemistry profile included calcium, phosphorus, sodium, potassium, chloride, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine level.
At the end of the experiment, autopsy was performed from the four most weight-reduced mice in calcitriol group and two in vehicle group and took major organs including liver, kidney, and spleen. Pathologic examination was done using both macroscopic and microscopic analysis. Major organs were examined in paraffin sections using a regular hematoxylin and eosin staining method.
Two sample t-test was employed to determine differences in the mean numbers of tumors and blood test levels between calcitriol and vehicle group. For assessment of histological tumor type, the Pearson's chi-squared test was used. In all analysis, PASW Statistics for Windows (version 18.0; IBM Co., Armonk, NY, USA) was used. Data were valued with in a confidence interval of 95%. A p-value of less than 0.05 was considered statistically significantly different (p<0.05).
The formation of first tumor could be observed between 18th and 20th weeks in both calcitriol group and vehicle group. After 21th week, tumor formation continuously increased. All mice showed tumor formation at 23rd week in vehicle group and 26th week in calcitriol group. Control group showed no tumor formation until the end of the study (Fig. 1).
Total tumor count in calcitriol group was lower than vehicle group throughout the experiment (Fig. 2A). In terms of tumor size, tumors less than 3 mm in size tended to be fewer by 2 to 5 in calcitriol group on average, statistically significant at 21st week however not significant in the following weeks (Fig. 2B). The number of tumors more than 3 mm in size was significantly fewer in calcitriol group after 25th week compared to vehicle group, with significantly decreased tumor formation rate which became more definite over time (Fig. 2C). These results suggested that the tumor formation was suppressed upon the application of topical calcitriol.
Representative biopsy sections of different types of tumors are presented in Fig. 3. On a murine carcinogenetic model, tumors progress from low grade malignancy (dysplastic papilloma) to high grade malignancy (poorly differentiated SCC and spindle cell SCC).
Pathological type of tumor in the experiment consisted of tumors from dysplastic papilloma to poorly differentiated SCC (Table 1). Six of nine samples in vehicle group showed high grade malignancy from moderately to poorly differentiated SCC, while 8 of 9 samples in calcitriol group showed low grade malignancy including dysplastic papilloma and keratoacanthoma. Although the limited number of samples did not show a statistically significant difference, tumors in calcitriol group showed a tendency to be histologically benign (p=0.061), while large sized tumors tended to show higher grade of malignancy. Since calcitriol group demonstrated less progressed stage of carcinogenesis than vehicle group, calcitriol may have a protective effect on tumor progression and therefore prevent the development of more aggressive cancer.
Upon immunofluorescence staining, the expression level of CPD in keratinocyte nucleus was lower in calcitriol group compared with vehicle group one hour after UV exposure. The difference became more prominent at eleven hours after UV exposure (Fig. 4). Despite the few sample numbers, this result indicated that topical application of vitamin D3 can protect from DNA damage induced by UV in accordance with previous reports14.
At 23rd week, the mean body weight of calcitriol treated, vehicle treated, and untreated control mice were 26.23±3.57 g, 29.53±1.87 g, and 29.34±1.25 g, respectively, with statistically significant difference between calcitriol group and other groups (p=0.004 and 0.017, respectively). The degree of weight loss in calcitriol group was greatest at 25th week, with the weight of 24.66±3.6 g in contrast to 29.49±2.0 g in vehicle group (p<0.001). When the weight loss reached up to 20% in calcitriol group, experiment was terminated at 26th week which was earlier than initially scheduled 30th week.
To elucidate the cause of weight loss, autopsy of vital organs and blood tests were done at the end of the study. On gross examination of internal organs, weight reduced mice in calcitriol group had little abdominal fat and slightly swollen kidney. Spleen was enlarged in some mice. Food was found in their stomach and therefore the weight loss was less likely to be due to starvation. Other internal organs including liver and lung showed normal appearance. On microscopic examination, three out of four calcitriol treated mice showed interstitial perivascular lymphocytic infiltration in kidneys, suggesting a mild nonspecific interstitial nephritis (Fig. 5). However, there was no calcification. Liver and spleen showed normal histological findings in all mice. Overall, the macro- and micro-morphology of major organs showed no specific pathological findings.
Serum levels of calcium and phosphate of calcitriol group were higher than vehicle group and were above normal range (Fig. 6). The levels of potassium and ALT in calcitriol group were significantly higher than vehicle group, but were within normal range. There were no abnormalities in other chemistry levels such as BUN, creatinine, sodium, chloride, AST and CBC including WBC, hemoglobin and platelet (data not shown). These data indicated that calcitriol treated mice was in a chronic hypercalcemic state.
Mutagenic DNA damage and local immunosuppression caused by UV irradiation are both necessary to induce skin cancers. There are number of types of DNA damage and its consequences, and among them CPD is one of the commonest detectable markers. If not repaired properly, it can result in UV "signature" mutations15. Oxidative damage, which results in the mutagen-prone base product 8-hydroxy-2-deoxyguanosine, may also play a role in UV-induced carcinogenesis.
It is known that 1,25(OH)2D has a photoprotective effect and is able to reduce UV-induced cell death, immunosuppression and mutagenic activity16. Recent studies specifically indicate that vitamin D signaling has a protective role in skin carcinogenesis through its corrective cellular responses to UVB irradiation–induced DNA damage1417181920. Cutaneous vitamin D system is known to be associated with sonic hedgehog signaling in the development of both benign and malignant epidermal neoplasm1821, and reduce UV-induced DNA damage in the form of CPD in mouse and human skin19.
Using murine UV-induced photocarcinogenesis model with SKH:hr-1 hairless mice, we investigated the effect of topical application of calcitriol on the development of skin tumors, and showed an example of skin cancer prevention. Recently there was a report which provided similar results with our study that topical vitamin D3 had inhibitory effect on UV-induced skin carcinogenesis using murine model22. Our study is different from the study in that we had longer duration of UV-exposure (20 weeks vs. 10 weeks), and had histopathologic analysis of representative tumors to not only reveal the effect on incidence of tumor formation but also investigate the effect of calcitriol on progression of tumors. Although this study is consistent with aforementioned researches, it may provide additional information in confirming both the preventive and adverse effects of topical treatment with calcitriol in the development of non-melanoma skin cancer. Our experiment showed that topical application of calcitriol reduced the number of tumors. Although not statistically significant throughout the study, total tumor count was less in calcitriol group than vehicle group, and tumors less than 3 mm in size may have significantly decreased in number in calcitriol group provided that the experiment could be continued for a longer period of time. Based on the results, we can speculate that topical calcitriol may have a role in reducing the rate of tumor formation. Furthermore, since tumors more than 3 mm in size were significantly affected, it suggests that the effect of topical calcitriol may also be based on delaying the progression of tumor development. Moreover, most of the tumors in calcitriol group were dysplastic papilloma, which are far less aggressive compared to those of vehicle group. Therefore, because topical calcitriol delayed the tumor's progression towards malignancy in this experiment, it may have role in suppressing the progression of tumor development. Our results are consistent with previous preliminary studies focused on potential of topical vitamin D derivatives in preventing skin cancer2324.
Recently it has been suggested that the vitamin D has photo-protective effect through vitamin D receptor (VDR), with or without its ligand 1,25(OH)2D, which limits the propensity for cancer formation following UV irradiation4. Active vitamin D functions via two major pathways, the classical steroid receptor (genomic) pathway or the rapid, putative membrane receptor (non-genomic) pathway19. Both pathways are known to be related with protection against DNA damage and carcinogenesis25. VDR is involved in both pathways and appears to have a set of mechanisms which do not require the regulation of its agonist, 1,25(OH)2D26. However, active vitamin D3 exerts photoprotective effects especially through non-genomic pathway by inducing DNA repair enzymes and regulating several genes responsible for cell proliferation via activation of VDR, which supports nucleotide excision repair (NER) activity and thereby reducing formation of CPD and result in improvement of several anti-cancer effects2142627. Results in our study showed that topical application of exogenously active agonist can be effective in reducing the formation of CPD (Fig. 4), and therefore we can presume that binding of an agonist to VDR may potentiate the action of VDR on cancer prevention. Furthermore, calcitriol has anti-cancer effects by repressing the expression of some members of the hedgehog signaling pathway which is related to epidermal tumor formation directly regulated by the VDR signaling18, and by enhancing pigmentation in melanocytes and increasing cornification in keratinocytes which are well known mechanisms of endogenous photoprotection in human skin1928. Our study, however, was not able to confirm these effects.
As for the adverse effects, weight loss of calcitriol group began from 23rd week and became more prominent at 25th week. This made the study end at 26th week which was initially planned to be ended at 30th week. Blood calcium and phosphate levels increased significantly upon the termination of study, and kidney biopsy showed interstitial nephritis. Serum level of vitamin D3, however, was not evaluated in this study. Chronic hypercalcemia is known to induce weight loss and interstitial nephritis, therefore weight loss in our experiment is likely to be from hypercalcemic state29. This is possibly due to relatively small total body surface area of mouse upon application of topical calcitriol which covered 40% of body area. Such possibility of hypercalcemia was also pointed out in previous study using murine model22. However, provided that topical calcitriol is used suitably such as intermittently applying on limited body surface area of human skin especially on face or other sun exposed areas only, the effect of hypercalcemia in human is less likely to be influential, and ultimately result in beneficial effect in preventing skin cancer. Moreover, a non-calcemic vitamin D analog may be developed in order to reduce hypercalcemic effect while maintaining anti-cancer effect. Topical application of vitamin D3 on patients with psoriasis undergoing frequent UV phototherapy may have a valuable effect in preventing possible skin carcinogenesis which can be induced by UV irradiation. Furthermore, since it has been reported that supplementing vitamin D in patients with atopic dermatitis may contribute to primary prevention of the disease, various combinations of indications on inflammatory skin diseases can be considered30.
In conclusion, topical application of calcitriol has shown protective effect against UV-induced non-melanoma skin cancer in a murine model. By utilizing the data of our study, further prospective study on UV-induced photocarcinogenesis in human model can be considered to verify the anti-cancer effect of topical calcitriol on human skin.
Figures and Tables
Fig. 1
Gross morphology of dorsum of mouse of each group at 25th week of experiment. Sham light control (A) showed no tumor formation, while vehicle group (B) and calcitriol group (C) showed tumor formation, with vehicle group showing numerous tumors in contrast to few tumors in calcitriol group.
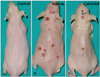
Fig. 2
Number of tumors in vehicle and calcitriol group between 21st and 26th week of experiment according to different tumor size. (A) Total tumor count (tumors of all size) was higher in vehicle group, without statistical significance except for 21st week. (B) Tumors of 1~3 mm in size were fewer by 2 to 5 on average in calcitriol group, statistically significant at 21st week however not significant thereafter. (C) Tumors more than 3 mm in size showed lower tumor formation rate in calcitriol group compared to vehicle group, with statistical significance at 25th and 26th week. *p<0.05.

Fig. 3
Histopathological findings of tumor types according to six categories of tumor progression at 26th week of experiment. Dysplastic papilloma with horn cysts: (A) the overall architecture is similar to papilloma with epidermal proliferation and horn cysts without prominent dermal invasion; (B) however it contains dysplastic cells in basal layer. Keratoacanthoma: (C) relatively infiltrative than dysplastic papilloma and individual keratinization begins to appear, (D) with some anaplastic cells. Well-differentiated squamous cell carcinoma (SCC): (E) more than 75% of the tumor is keratinized, (F) with slight amount of anaplastic cells. Moderately differentiated SCC: (G) between 25% and 75% of the tumor is keratinized, (H) with moderate amount of anaplastic cells. Poorly differentiated SCC: (I) less than 25% of the tumor is keratinized, (J) with relatively abundant anaplastic cells. H&E; A, C, E, G, I: ×100; B, D, F, H, J: ×400. Diff.: differentiated, mod.: moderately.
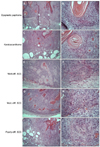
Fig. 4
Immunofluorescence detection of cyclobutane pyrimidine dimer (CPD) at 1 and 11 hours after ultraviolet-exposure. (A) Vehicle group, (B) calcitriol group. CPD positive cells (yellow-green fluorescence, white arrows) are relatively fewer in calcitriol group than vehicle group (red fluorescence: propidium iodide staining of nuclei). A, B: ×400.
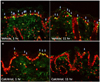
Fig. 5
Kidney biopsy of mouse in calcitriol group at 26th week of experiment showing non-specific interstitial nephritis. Dense lymphohistiocytic inflammatory cellular infiltrates in interstitial and tubular area (A) and relatively few infiltrates near glomerulus (B). (A, B) H&E, ×200.
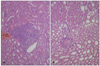
Fig. 6
Laboratory results of vehicle and calcitriol group at 26th week of experiment. In calcitriol group, significantly increased level of calcium (normal range: 7.1~10.1 mg/dl) (A), phosphate (normal range: 5.7~9.2 mg/dl) (B), potassium (normal range: 5~7.5 mEq/L) (C), and ALT (normal range: 17~77 U/L) (D) could be seen, suggesting a hypercalcemic state. AST: alanine aminotransferase.

ACKNOWLEDGEMENT
This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (2012K1A4A3053142).
References
2. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev. 2012; 33:456–492.

3. Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008; 128:2880–2887.

4. Bikle DD. Protective actions of vitamin D in UVB induced skin cancer. Photochem Photobiol Sci. 2012; 11:1808–1816.

5. Bikle DD, Elalieh H, Welsh J, Oh D, Cleaver J, Teichert A. Protective role of vitamin D signaling in skin cancer formation. J Steroid Biochem Mol Biol. 2013; 136:271–279.

6. Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979; 2:1335–1336.
7. Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 2008; 624:31–42.

8. Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol. 2012; 67:803.e1–803.e12. quiz 815-816.
9. Lerche CM, Philipsen PA, Poulsen T, Wulf HC. Topical hydrocortisone, clobetasol propionate, and calcipotriol do not increase photocarcinogenesis induced by simulated solar irradiation in hairless mice. Exp Dermatol. 2010; 19:973–979.

10. Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Topical treatment with diclofenac, calcipotriol (vitamin-D3 analog) and difluoromethylornithine (DFMO) does not prevent nonmelanoma skin cancer in mice. Cancer Invest. 2013; 31:92–96.

11. Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009; 4:1350–1362.

12. Kusewitt DF, Applegate LA, Ley RD. Ultraviolet radiation-induced skin tumors in a south american opossum (monodelphis domestica). Vet Pathol. 1991; 28:55–65.

13. Gye J, Ahn SK, Kwon JE, Hong SP. Use of fractional CO2 laser decreases the risk of skin cancer development during ultraviolet exposure in hairless mice. Dermatol Surg. 2015; 41:378–386.

14. Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J Invest Dermatol. 2012; 132:2097–2100.

15. Rochette PJ, Therrien JP, Drouin R, Perdiz D, Bastien N, Drobetsky EA, et al. UVA-induced cyclobutane pyrimidine dimers form predominantly at thymine-thymine dipyrimidines and correlate with the mutation spectrum in rodent cells. Nucleic Acids Res. 2003; 31:2786–2794.

16. Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, et al. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005; 97:137–143.

17. Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008; 128:2508–2517.

18. Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011; 131:2289–2297.

19. Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1alpha, 25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010; 121:164–168.

20. Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, et al. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009; 458:505–508.

21. Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev Res (Phila). 2011; 4:744–751.

22. Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, et al. 1α,25(OH)2-vitagmin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila). 2011; 4:1485–1494.

23. Kensler TW, Dolan PM, Gange SJ, Lee JK, Wang Q, Posner GH. Conceptually new deltanoids (vitamin D analogs) inhibit multistage skin tumorigenesis. Carcinogenesis. 2000; 21:1341–1345.

24. Wood AW, Chang RL, Huang MT, Uskokovic M, Conney AH. 1 alpha, 25-dihydroxyvitamin D3 inhibits phorbol ester-dependent chemical carcinogenesis in mouse skin. Biochem Biophys Res Commun. 1983; 116:605–611.

26. Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2014; 148:47–51.

27. Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007; 103:451–456.

28. Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988; 91:593–598.

29. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009; 3:71–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download