Abstract
Background
Mannitol containing monophasic filler with higher crosslinking has not been well studied for moderate and severe nasolabial fold (NLF) correction.
Objective
To compare the efficacy and safety of a novel mannitol containing hyaluronic acid (HA) filler (HA-G) with biphasic HA filler (HA-P) for moderate and severe NLF correction.
Methods
Thirteen subjects with symmetric moderate to severe NLF received HA-G (in one NLF) and HA-P (in other NLF) and were evaluated for 24 weeks.
Results
At both 12 and 24 weeks, the mean improvement in Genzyme 6-point grading scale from baseline was significantly greater in the side of face that was treated with HA-G than HA-P (1.96±0.91 vs. 1.54±0.73 at week 12; p=0.044, 1.88±0.78 vs. 1.3±0.79 at week 24; p=0.027, respectively). At 12 weeks, the mean Global Aesthetic Improvement Scale score was 2.92±0.93 for HA-G and 2.31±0.95 for HA-P (p=0.008). Both fillers were well tolerated.
There is increasing realization that minimally invasive procedures for facial rejuvenation create a younger, healthier, and happier look. Among those procedures, filler injection has been used extensively, and a nasolabial fold (NLF) is one of the most popular target areas for wrinkle correction and soft tissue augmentation with filler alone or in combination with other treatment modalities such as radiofrequency therapy1. The ideal filler would be long lasting, have minimal side effects, and be easy to inject2. Currently, hyaluronic acid (HA) comprises the largest group of fillers currently available due to its long duration and low immunogenic potential3. HA is strongly hydrophilic, binds readily to water molecules and swells to form an extended matrix that occupies a large volume4. In addition, it has been suggested that HA stimulates collagen synthesis and inhibits collagen degradation, which may contribute to the long duration of its effects5. However, native HA polymer chains alone would demonstrate only a limited durability with a half-life of 1 to 2 days because they would be rapidly degraded by hyaluronidases and free radicals, which are present in the dermis.
As a consequence, there have been many attempts to modify HA to improve its physical and chemical properties including durability and cross-linking. Adding excipients could also be an effective method to enhance the rheologic properties of hyaluronic fillers also.
A novel mannitol containing monophasic HA filler (HA-G; Glytone 3®, Merz Pharmaceuticals GmbH, Frankfurt, Germany) is a product with a distinct compositional feature. HA-G contains a HA content of 23 mg/ml with 85% cross-linking. With a higher concentration of HA and higher proportion of cross-linking, HA-G is expected to have enhanced durability. More importantly, 41 mg of mannitol is added in a 1 ml syringe. Mannitol is derived from sugar (mannose) by reduction, and is commonly used and effective pharmaceutical binder. The high physiological tolerability and chemical inertness of mannitol make it an excellent excipient for pharmacologic formulations. It does not pick up water from the air until the humidity level reaches 98%6, and when injected intravenously, it decreases the blood viscosity7. It is also known for free radical scavenger.
These physicochemical properties of mannitol above would make HA fillers more suitable for rejuvenation. Mannitol would be expected to increase the durability of HA without foreign body reaction, and make injections easier.
Accordingly, we designed a randomized, evaluator-blind, split-face study to measure the efficacy and safety of HA-G for the treatment of moderate and severe NLFs compared with Perlane® (HA-P; Q-Med AB, Uppsala, Sweden). HA-P was also used as a comparison product to demonstrate the efficacy and safety of novel fillers as indicated in many previous studies8910. HA-P is a biphasic injectable filler with an HA content of 20 mg/ml and that is composed of 80% cross-linked HA. Both HA-G and HA-P use 1,4-butanediol diglycidal ether as the cross-linker and were developed for injection into facial wrinkles ranging from average to deep.
This was a randomized, evaluator-blinded, split-face comparison study conducted at the Asan Medical Center and a private clinic (Seoul, Republic of Korea) between August 2011 and August 2012. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and local regulatory requirements. The study was reviewed and approved by the institutional review board of Asan Medical Center (S2014-0775). All patients provided written informed consent prior to enrollment. Healthy male and female patients were eligible to participate in this study if they had NLFs on both sides with a rating of grade 3 (moderate) or higher according to the Genzyme 6-point grading scale (GGS)11. Exclusion criteria included the receipt of any kind of filler injection or surgery on NLF during the previous 24 months. Women who were pregnant, nursing, or planning a pregnancy were also excluded. During the study period, any procedure or treatment that might affect the evaluation of the NLF or the area around the mouth was not permitted, with the exception of topical skincare products.
HA-G and HA-P were used in this study (Table 1). Each participant received HA-G in one NLF and HA-P in the other NLF. A mixture of 2.5% lidocaine and 2.5% prilocaine ointment (Emla®; AstraZeneca, Karlskoga, Sweden) was applied at least 45 minutes before injection. The cream was removed before injection, and a routine non-alcoholic aseptic technique was used. Both products were injected into the mid-dermis using a 27-gauge needle, with the aim of achieving a 100% correction of the NLF with no overcorrection. The linear threading and serial puncture technique were used for injection. The administered volume was determined at the discretion of the injector, with up to 2 ml at baseline treatment and up to 1 ml for touch-up for both products. A touch-up injection was administered if deemed to be necessary two weeks after baseline treatment. Follow-up examinations were performed at baseline, and at 4, 12, and 24 weeks after the initial treatment. Photographs of each subject were taken at every visit. An independent panel of three dermatologists made assessments using these photographs only (no additional information was provided) and their scores were averaged. To undertake the study in a blind manner, the practitioner who administered the injections did not participate in any of the efficacy evaluations. The following outcome parameters were evaluated for efficacy: changes in the GGS score according to the independent expert panel using standardized photographs, changes in the Global Aesthetic Improvement Scale (GAIS) according to the expert panel, and participant preference between the two fillers tested after the study had completed. Changes were calculated at 4, 12, and 24 weeks from baseline.
The assessment of wrinkles was performed using the 6-point GGS (0, no wrinkles; 1, just perceptible; 2, shallow; 3, moderately deep; 4, deep; 5, well-defined folds; and 6, very deep, redundant folds). A clinically meaningful treatment effect was defined as a reduction of one point or more on the GGS score. The GAIS values are 0 (no change), 1 (slightly improved), 2 (improved), 3 (much improved), and 4 (very much improved). At the end of the study, the subjects were asked to choose which side of the NLF they preferred in terms of overall treatment effects. Tolerability was assessed based on each subject's diary. For the assessment of safety, injection site reactions and adverse events were noted at every visit.
All data were summarized and analyzed using SAS version 9.1 (SAS Institute, Cary, NC, USA). A p-value<0.05 was used to determine statistical significance. Improvements in GGS scores were compared with the baseline using a linear mixed effects model. The proportion of NLFs that demonstrated great improvement (two or more score reduction on the GGS compared with baseline) was evaluated using generalized estimating equations.
A total of 13 subjects were enrolled in these analyses. The demographic characteristics were summarized in Table 2. No subjects had a baseline GGS score of lower than 3 for either NLFs. Baseline GGS scores between both sides of the NLFs showed no significant difference (p=0.22). Two fillers were randomly assigned to the right or left NLF. The mean administered volume of HA-G was 0.64±0.29 ml and of HA-P was 0.68±0.23 ml which was not significantly different (p=0.37). Touch-up injections were conducted for 9 sides for HA-G and 8 sides for HA-P. The mean touch-up volume of HA-G was 0.22±0.02 ml and HA-P was 0.21±0.03 ml (p=0.78).
The mean GGS score at 4 weeks decreased by 2.21 on the HA-G treated side (from 3.5 at baseline to 1.29 at 4 weeks after treatment), and by 1.85 for the HA-P treated side (from 3.05 at baseline to 1.2 at 4 weeks after treatment) (Fig. 1). When evaluated again at 12 and 24 weeks after treatment, the improvement was maintained. The mean decrease in the GGS score from baseline was 1.96 for HA-G, and 1.54 for HA-P at 12 weeks, and 1.88 for HA-G and 1.3 for HA-P at 24 weeks. Differences between the GGS score decreases of the two fillers showed statistical significance (p=0.044 at 12 weeks and 0.027 at 24 weeks). The proportion of NLFs showing great improvement was 69.2% for the HA-G treated side and 61.5% for the HA-P treated side at 4 weeks after treatment and 61.5% for the HA-G treated side and 38.4% for the HA-P treated side at 12 weeks after treatment (Fig. 2).
Four weeks after treatment, the mean GAIS score was 3±1 for HA-G and 2.77±1.04 for HA-P. At 12 weeks, these scores were 2.92±0.93 for HA-G and 2.31±0.95 for HA-P (Fig. 3). The p-values for the differences between the HA-G vs. HA-P scores were 0.337 at 4 weeks and 0.008 at 12 weeks.
At 12 weeks after treatment, participants could choose which side they preferred. Participants could also elect no preference if they thought there was no difference. Three out of 13 subjects preferred HA-G and 10 subjects had no preference. The reasons for indicating a preference included subjective impressions of better skin texture at the injection site and longevity.
Generally, the safety of both fillers was tolerable and no serious adverse events occurred during the study. Only one patient with bruising at the injection site was observed for each HA-G and HA-P treatment, respectively. All cases of adverse events were temporary and tolerable in our current series. There was no need for any secondary treatment and they didn't last longer than 4 weeks. Erythema and pain at the injection site which persisted for less than 3 days were not counted as adverse events. None of our subjects complained of severe pain or discomfort during the procedure for either filler.
Herein we compared the novel mannitol-containing HA dermal filler with HA-P. These two fillers had a number of different properties including type, HA concentration, and the degree of cross-linking of HA. However, even if they had had identical properties, there were a number of other factors that would have affected their performance such as the gel-to-fluid ratio and the degree of HA modifications that were determined by manufacturing process121314. Thus, it was not really possible to match all of the factors individually and we instead compared products which were used largely for the same purposes to demonstrate their efficacy and safety. This approach is consistent with previous studies that have compared dermal fillers. HA-P is one of the dermal HA fillers which has been used widely for the augmentation of deep wrinkles such as NLFs and has a proven efficacy and safety8910.
In our present study, 2 rubrics, the GGS and GAIS, were used to compare efficacy of 2 filler materials. Based on the GGS, the HA-G filler demonstrated a higher treatment efficacy profile to HA-P in the treatment of moderate and severe NLF during 24 weeks of follow-up (Fig. 1, 4, and 5). At 4 weeks after treatment, the proportion of subjects who showed great improvement on the HA-G treatment side was higher than that of the HA-P treated side group, but this was not statistically significant (p=0.059). At 12 and 24 weeks after treatment, the mean GGS improvement on the HA-G treated side was higher than that of the HA-P treated side, with statistical significance. Consistent with the observed GGS change profile, the GAI scores were not significantly different at week 4, but a better durability was noted on the HA-G treated side at 12 weeks after treatment.
The distinct feature of HA-G is that it contains mannitol which is believed to have anti-oxidant properties. Many studies have reported the therapeutic and preventative effects of mannitol against ischemic injury because it acts as an oxygen free radial scavenger15161718. Most notably, a previous study of ophthalmic visco-surgical devices has reported that the addition of mannitol to HA produced protective effects against free radicals19. The elimination of HA in the dermis consists of an enzymatic degradation in the liver where free radicals are essential cofactors. Hence, we expected that the addition of mannitol would maintain HA for a longer period within the dermal tissue, at least in part by attenuating free radical activities. Our current results indicate, on the basis of mean reduction in the GGS score at 12 and 24 weeks after treatment, that HA-G shows better persistence than HA-P. The mean reduction in the GGS score at 24 weeks after treatment was 1.88 for HA-G and 1.3 for HA-P, a statistically significant difference (p=0.027). This result demonstrates that HA-G has a higher longevity than HA-P.
Both the HA-G and HA-P fillers demonstrated excellent safety and tolerability in our hands. Swelling and edematous changes at the injection sites were anticipated given the osmotic properties of mannitol. However, only one of our current patient subjects reported bruising at the HA-G treated side, which was not related to swelling.
Owing to its lower elastic modulus, the novel HA-G filler was easier to inject into the appropriate target tissue. During the injection procedure there was no formation of 'bumps' at the injection site, which is common when using conventional HA based fillers. Additionally, the high level of HA cross-linking endowed resilience to the HA-G filler, retaining structural integrity after dermal injection.
To the best of our knowledge, there have been only two reports that evaluated the efficacy and safety of mannitol-containing fillers. This product showed significant effects on skin elasticity and complexion radiance implying that this occupied a large volume. Also, no severe adverse events occurred2021.
Although some fillers have been studied and compared previously, their efficacy is highly subjective as it is based on the physician's impression and patient's satisfaction. The absence of a well established and standardized rating scale for wrinkles might be one of the reasons for this subjectivity. The GGS system was designed to overcome the shortcomings of previous wrinkle assessment tools for the treatment of NLFs. This system improved on existing rating score scales22 through its use of standardized same face wrinkle grading photographs and provides more subdivided criteria by using 6 point grading for more elaborate comparisons. We could therefore provide a reliable NLF improvement assessment in our current analysis using GGS.
This study had some limitations to note. First, the number of subjects was too small to demonstrate efficacy of the filler with adequate statistical power. Future studies of larger cohorts will thus be needed. Second, we conducted evaluations for up to 24 weeks only. A longer-term assessment of efficacy and safety is warranted.
In summary, we find that HA-G shows a superior treatment efficacy profile to HA-P in the treatment of moderate and severe NLF during a 24 week follow-up period. HA-G could therefore be added to the existing group of dermal fillers whose efficacy and safety have been verified in prospective clinical trials. This provides practitioners with an additional choice for soft tissue augmentation, especially NLFs.
Figures and Tables
 | Fig. 1Mean improvement from baseline in Genzyme 6-point grading scale score based on assessments by evaluating experts (*p≤0.05). HA: hyaluronic acid, HA-G, Glytone 3® (Merz Pharmaceuticals GmbH, Frankfurt, Germany); HA-P, Perlane® (Q-Med AB, Uppsala, Sweden). |
 | Fig. 2Proportion of subjects who showed great improvement (≥2 reduction of Genzyme 6-point grading scale score). HA: hyaluronic acid, HA-G, Glytone 3® (Merz Pharmaceuticals GmbH, Frankfurt, Germany); HA-P, Perlane® (Q-Med AB, Uppsala, Sweden). |
 | Fig. 3Mean GAIS score at 4 weeks and 12 weeks after treatment (*p≤0.05). HA: hyaluronic acid, HA-G, Glytone 3® (Merz Pharmaceuticals GmbH, Frankfurt, Germany); HA-P, Perlane® (Q-Med AB, Uppsala, Sweden), GAIS: global aesthetic improvement scale. |
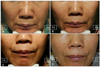 | Fig. 4Subject (61 years/female) with dermal injection of HA-P in right nasolabial fold (NLF) and HA-G in left NLF. Genzyme 6-point grading scale change from baseline to 24 weeks. Right: HA-P, 3.6 to 2.3; left: HA-G, 3.3 to 0.3. (A) Baseline, (B) 4 weeks, (C) 12 weeks, and (D) 24 weeks after treatment. HA: hyaluronic acid, HA-G, Glytone 3® (Merz Pharmaceuticals GmbH, Frankfurt, Germany); HA-P, Perlane® (Q-Med AB, Uppsala, Sweden). |
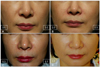 | Fig. 5Subject (42 years/female) with dermal injection of HA-G in right nasolabial fold (NLF) and HA-P in left NLF. Genzyme 6-point grading scale changed from baseline to 24 weeks. Right: HA-G, 3.6 to 0.3; left: HA-P, 3 to 0.6. (A) Baseline, (B) 4 weeks, (C) 12 weeks, and (D) 24 weeks after treatment. HA: hyaluronic acid, HA-G, Glytone 3® (Merz Pharmaceuticals GmbH, Frankfurt, Germany); HA-P, Perlane® (Q-Med AB, Uppsala, Sweden). |
Table 1
Characteristics of the target fillers in this study

| Characteristic | HA-G | HA-P |
|---|---|---|
| Type | Monophasic | Biphasic |
| HA concentrates (mg/ml) | 23 | 20 |
| Composition of uncross-linked HA (%) | 15 | 20 |
| Cross-linker | BDDE | BDDE |
References
1. Kim H, Park KY, Choi SY, Koh HJ, Park SY, Park WS, et al. The efficacy, longevity, and safety of combined radiofrequency treatment and hyaluronic acid filler for skin rejuvenation. Ann Dermatol. 2014; 26:447–456.

2. Gold M. The science and art of hyaluronic acid dermal filler use in esthetic applications. J Cosmet Dermatol. 2009; 8:301–307.

3. Andre P. Hyaluronic acid and its use as a "rejuvenation" agent in cosmetic dermatology. Semin Cutan Med Surg. 2004; 23:218–222.

4. Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: scientific and technical considerations. Plast Reconstr Surg. 2007; 120:33S–40S.

5. Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007; 143:155–163.

6. Ohrem HL, Schornick E, Kalivoda A, Ognibene R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm Dev Technol. 2014; 19:257–262.

7. Andrews RJ, Bringas JR, Muto RP. Effects of mannitol on cerebral blood flow, blood pressure, blood viscosity, hematocrit, sodium, and potassium. Surg Neurol. 1993; 39:218–222.

8. Goodman GJ, Bekhor P, Rich M, Rosen RH, Halstead MB, Rogers JD. A comparison of the efficacy, safety, and longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: a multicenter, prospective, randomized, controlled, single-blind, withinsubject study. Clin Cosmet Investig Dermatol. 2011; 4:197–205.

9. Ascher B, Bayerl C, Brun P, Kestemont P, Rzany B, Poncet M, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines-6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011; 10:94–98.

10. Nast A, Reytan N, Hartmann V, Pathirana D, Bachmann F, Erdmann R, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011; 37:768–775.

11. Monheit GD, Gendler EC, Poff B, Fleming L, Bachtell N, Garcia E, et al. Development and validation of a 6-point grading scale in patients undergoing correction of nasolabial folds with a collagen implant. Dermatol Surg. 2010; 36:Suppl 3. 1809–1816.

12. Edsman K, Nord LI, Ohrlund A, Larkner H, Kenne AH. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012; 38:1170–1179.

13. Falcone SJ, Berg RA. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res A. 2008; 87:264–271.

14. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009; 35:Suppl 1. 302–312.

15. England MD, Cavarocchi NC, O'brien JF, Solis E, Pluth JR, Orszulak TA, et al. Influence of antioxidants (mannitol and allopurinol) on oxygen free radical generation during and after cardiopulmonary bypass. Circulation. 1986; 74:III134–III137.
16. Weinbroum AA. Mannitol prevents acute lung injury after pancreas ischemia-reperfusion: a dose-response, ex vivo study. Lung. 2009; 187:215–224.

17. Liu JH, Wann H, Chen MM, Pan WH, Chen YC, Liu CM, et al. Baicalein significantly protects human retinal pigment epithelium cells against H2O2-induced oxidative stress by scavenging reactive oxygen species and downregulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor. J Ocul Pharmacol Ther. 2010; 26:421–429.

18. Khoury W, Namnesnikov M, Fedorov D, Abu-Gazala S, Weinbroum AA. Mannitol attenuates kidney damage induced by xanthine oxidase-associated pancreas ischemia-reperfusion. J Surg Res. 2010; 160:163–168.

19. Belda JI, Artola A, Garcia-Manzanares MD, Ferrer C, Haroun HE, Hassanein A, et al. Hyaluronic acid combined with mannitol to improve protection against free-radical endothelial damage: experimental model. J Cataract Refract Surg. 2005; 31:1213–1218.

20. Baspeyras M, Rouvrais C, Liegard L, Delalleau A, Letellier S, Bacle I, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013; 305:673–682.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download