Dear Editor:
Sebaceous hyperplasia (SH) is a benign proliferation of the sebaceous glands that develops as a result of intrinsic aging, photo-aging, exposure to ultraviolet radiation, and genetic predisposition. Organ transplant recipients are susceptible to SH particularly on the face, where multiple large lesions may cause cosmetic concern. Several studies in the literature report SH being induced by cyclosporine due to immunosuppression of patients undergoing organ transplant. Oral isotretinoin and topical photodynamic therapy has previously been reported as a treatment for SH in immunocompetent individuals123. Other options include electrodesiccation, shave excision and carbon dioxide laser. However, only three cases of cyclosporine-induced SH in a kidney transplant population treated with oral isotretinoin have been reported in the English literature23. Therefore, we report a case of cyclosporine-induced SH in a renal transplant recipient successfully treated with oral isotretinoin, along with a review of the literature.
A 40-year old Korean man presented in 2013 with multiple skin-colored papules on his face. He had undergone renal transplantation surgery in 1996, following which, he was immunosuppressed with oral cyclosporine and prednisolone for 11 years. He started to notice the facial lesions during this period. The initial doses were cyclosporine 350 mg (5.0 mg/kg body weight) and prednisolone 7.5 mg. The initial dosage was tapered and the patient eventually stopped taking the immunosuppressants in 2007. The patient then started taking tacrolimus 6 mg and prednisolone 5 mg for 4 years. Examination of his face revealed approximately a hundred skin-colored, umbilicated papules, 2 to 4 mm in size, which had increased in number for several years (Fig. 1A). Histological examination of the lesion demonstrated a peculiar superficial localization of the enlarged sebaceous glands in the upper dermis. The patient was diagnosed with SH. He was treated with daily oral isotretinoin 20 mg (0.29 mg/kg) for two months which resulted in near-complete remission without any side effect (Fig. 1B). Two months after discontinuing oral isotretinoin, a few new lesions started to develop. Oral isotretinoin 20 mg was restarted for two months. The lesions cleared without recurrence for nine months.
Cyclosporine-induced SH has been reported in several series of organ transplant recipients. Among renal transplant recipients, 7 out of 67 (10%) and 5 out of 82 patients (6%) developed SH. In all cases, the patients were taking cyclosporine4. In an earlier report of 151 kidney transplant recipients who were not taking cyclosporine, SH did not occur5. These studies suggest that cyclosporine is a direct and causative factor of SH. Both tacrolimus and cyclosporine are calcineurin inhibitors sharing similar physicochemical properties and a common mechanism of action - inhibition of proinflammatory cytokines. In addition, both drugs are highly soluble in lipids allowing for cutaneous accumulation of the drugs and leading to dysplastic epithelial proliferation. Therefore, in this patient, it is possible to that oral tacrolimus might have played a role in developing SH.
Sebaceous glands are mostly considered by dermatologists in association with acne and seborrhea; thus, the antiproliferative and sebostatic effects of isotretinoin have been extensively studied. Isotretinoin causes cell cycle arrest in human staphylococcal enterotoxin B-1 sebocytes in vitro, and apoptosis in human sebaceous glands in vivo2. To our knowledge, there are two other reports of renal transplant recipients receiving treatment for SH with isotretinoin (Table 1)23. Other reported effective treatments for cyclosporine-induced SH in patients undergoing renal transplant include carbon dioxide laser and topical photodynamic therapy.
In conclusion, our case provides additional support for the safe and effective use of oral isotretinoin for cyclosporine-induced SH in patients undergoing renal transplant. However, further studies are needed to establish a treatment protocol for cyclosporine-induced SH in patients undergoing renal transplant.
Figures and Tables
Fig. 1
(A) Prominent multiple hyperplastic sebaceous glands seen as skin-colored papules (arrows) scattered on the right side of the face after treatment of cyclosporine for 11 years and tacrolimus for 4 years. (B) Sebaceous hyperplasia almost completely cleared after 2 months of oral isotretinoin.
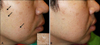
Table 1
Summary of previously reported cases of kidney transplant recipient receiving treatment for sebaceous hyperplasia with isotretinoin

| Author | Age (yr)/sex | Treatment regimen | Treatment duration until clearance | Side effect | Relapse |
|---|---|---|---|---|---|
| Burton et al.3 | 39/M | Isotretinoin 40 mg twice daily | 2 weeks | None | 3 weeks |
| McDonald et al.2 | 56/M | Isotretinoin 10 mg daily | 3 months | Minor cheilitis | Not stated |
| 45/M | Isotretinoin 20 mg daily | 4 months | None | ||
| Our patient | 40/M | Isotretinoin 20 mg daily | 2 months | None | 2 months |
ACKNOWLEDGMENT
This work was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2015R1C1A2A01055073).
References
1. Perrett CM, McGregor J, Barlow RJ, Karran P, Proby C, Harwood CA. Topical photodynamic therapy with methyl aminolevulinate to treat sebaceous hyperplasia in an organ transplant recipient. Arch Dermatol. 2006; 142:781–782.

2. McDonald SK, Goh MS, Chong AH. Successful treatment of cyclosporine-induced sebaceous hyperplasia with oral isotretinoin in two renal transplant recipients. Australas J Dermatol. 2011; 52:227–230.

3. Burton CS, Sawchuk WS. Premature sebaceous gland hyperplasia: successful treatment with isotretinoin. J Am Acad Dermatol. 1985; 12:182–184.

4. Lugo-Janer G, Sánchez JL, Santiago-Delpin E. Prevalence and clinical spectrum of skin diseases in kidney transplant recipients. J Am Acad Dermatol. 1991; 24:410–414.

5. Taylor AE, Shuster S. Skin cancer after renal transplantation: the causal role of azathioprine. Acta Derm Venereol. 1992; 72:115–119.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download