Dear Editor:
Fried et al.1 reported on a specific disease entity using the term "Pretibial lymphoplasmacytic plaque in children".
Herein, we describe a 3-year-old girl with a 6-month history of a solitary, asymptomatic, erythematous, scaly plaque with some papules over the right knee (Fig. 1A). She had a clean medical history with no history of travel abroad, injury, or insect bite. The biopsy specimen was characterized by focal parakeratosis, irregular acanthosis, and dense dermal lymphoid infiltrates, admixed with numerous plasma cells. The plasma cells extended into the deep dermis with a perivascular and periadnexal pattern, as previously reported by Ahn et al.2. The specimen also revealed a lichenoid reaction with basal vacuolization and lymphoid exocytosis, similar to those reported by Moulonguet et al.3 and Porto et al.4 (Fig. 1B, C).
The immunohistochemical studies showed mixed inflammation of the B-cell and T-cell lymphocytes. Additionally, a polyclonal pattern was confirmed by the co-existence of kappa and lambda light chain-positive cells. No organisms were identified on the acid fast bacilli, Giemsa, and periodic acid-Schiff staining. A serologic test for syphilis and the QuantiFERON-TB Gold (Cellestis Limited, Carnegie, Australia) test both yielded negative results.
Lymphoplasmacytic plaque (LPP) was originally termed "isolated benign primary cutaneous plasmacytosis in children," as it was thought to be a part of the spectrum of primary cutaneous plasmacytosis (PCP)5. Several similar cases, which have been reported as LPP or under a different diagnosis, shared common clinical features including a solitary plaque with a predilection for the lower legs and prevalence in the pediatric age group4. These features were distinct from those of PCP, which is characterized by multiple red-brown plaques located on the trunk, mainly in adult Asian patients1. These previously reported cases, which we believe represent LPP, are described in Table 1. Histopathologically, LPP exhibited an irregular acanthosis with overlying parakeratosis and dermal lymphoplasmacytic infiltrates, with or without epitheloid granulomas. The localized inflammation and epidermal hyperplasia may contribute to the papulosquamous morphology of LPP. Clinically, the plaque seemed to be a benign chronic condition, although there was no effective treatment other than complete excision (Table 1).
Moulonguet et al.3 differentiated LPP from acral pseudolymphomatous angiokeratoma of children, suggesting that these disease entities may reside on the spectrum of pseudolymphomas. Pseudolymphomas can develop after exposure to certain antigens. Interestingly, the histological pattern of a lichenoid inflammation with periadnexal infiltrate was very similar to those of lichen striatus, except for the heavy composition of plasma cells that is important for the diagnosis of LPP. Therefore, given the similarities in the age of onset and the histologic findings, we believe both conditions could be caused by a local reaction to an unknown, acquired stimulus in a specific age group.
In conclusion, although the pathophysiology is still unknown, LPP is a unique disease entity with typical clinical features. When a child presents with this type of plaque on the lower extremities, several disease entities including primary lymphoproliferative disorders, infectious lesions, other reactive infiltrates, or pseudolymphomas need to be considered. A final punch biopsy is necessary to confirm the diagnosis.
Figures and Tables
Fig. 1
(A) Solitary reddish scaly plaque on the right knee. (B) Punch biopsy shows dense inflammatory infiltrate in lichenoid arrangement with perivascular and periadenxal extension into the deep dermis (H&E, ×40). (C) Higher magnification revealing the infiltrate is composed of numerous plasma cells admixed with lymphocytes (H&E, ×400).
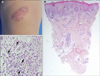
Table 1
Previously reported cases showing the same findings with lymphoplasmacytic plaque in children

| Study | Gender/age (yr) | Ethnicity | Location | Duration | Clinical appearance | Treatment |
|---|---|---|---|---|---|---|
| Gilliam et al.5* | F/15 | Caucasian | Pretibial | 11 years | 4.5 cm reddish brown violaceous plaque | Pulse-dye laser with partial improvement of the color |
| M/7 | Caucasian | Pretibial | 2 years | Cluster of dark reddish brown papulonodules | ||
| Fried et al.1 | F/11 | Caucasian | Pretibial | 5 years | 4.0 cm reddish brown plaque | Intralesional steroid injectionscwith partial remission |
| Ahn et al.2* | F/7 | Asian | Buttock | 7 years | 3.0 cm erythematous scaly plaque | Topical application of methylprednisolone acetate and tacrolimus hydrate and intralesional steroid injections with partial remission Eventually treated with excision |
| Moulonguet et al.3 | F/5 | Caucasian | Pretibial | 10 months | 3.5 cm reddish scaly plaque | Topicalsteroids (Clobetasol under occlusion) with slight improvement |
| M/11 | North African | Pretibial | 1 year | 3.0 cm reddish scaly plaque | Topical steroids with slight improvement | |
| Porto et al.4 | F/17 | Caucasian | Thigh | >10 years | 2.0 cm pink serpiginousplaque | Excision and recurrence free |
| F/2 | Caucasian | 3rd finger | 2 years | 8 mm erythematous scalyplaque | Observe the lesion while weighing treatment options | |
| Present case | F/3 | Asian | Knee | 6 months | 2.5 cm reddish scaly plaque | Topical steroids (0.3% Diflucortolone valerate ointment) with unsuccessful response and no change in size for a year |
References
1. Fried I, Wiesner T, Cerroni L. Pretibial lymphoplasmacytic plaque in children. Arch Dermatol. 2010; 146:95–96.

2. Ahn JJ, Yang YS, Shin MK, Lee SW, Kim NI. Case of isolated benign primary cutaneous plasmacytosis in a child. J Dermatol. 2011; 38:364–367.

3. Moulonguet I, Hadj-Rabia S, Gounod N, Bodemer C, Fraitag S. Tibial lymphoplasmacytic plaque: a new, illustrative case of a recently and poorly recognized benign lesion in children. Dermatology. 2012; 225:27–30.

4. Porto DA, Sutton S, Wilson JB, Scupham RK, Stone MS, Liu V. Lymphoplasmacytic plaque in children: a report of two new cases with review of the literature. J Cutan Pathol. 2013; 40:50–55.

5. Gilliam AC, Mullen RH, Oviedo G, Bhatnagar R, Smith MK, Patton DF, et al. Isolated benign primary cutaneous plasmacytosis in children: two illustrative cases. Arch Dermatol. 2009; 145:299–302.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download