Abstract
Onychomatricoma is a rare tumor of the nail matrix. Until now, few cases of onychomatricoma have been reported in the literature. Immunohistochemically, CD10, a marker of the onychodermis, is expressed in the stroma of the onychomatricoma. In the present case, a 27-year-old woman presented with an 8-year history of a yellowish, thickened, and overcurved nail plate of the right index finger, mimicking onychomycosis. She had been treated for 4 years with antifungal agents by general physicians, without improvement. The nail was surgically removed, and the tumor at the nail matrix was excised. The nail plate continued to grow in the 2 months after the excision. This is a case of onychomatricoma in South Korea, which was initially misdiagnosed as onychomycosis. In addition, we present a review of the literature regarding clinical, sonographic, and histological features, differential diagnoses, and treatment of onychomatricoma.
Baran and Kint1, is a rare benign tumor of nail matrix origin. Since its description, no more than 80 cases have been reported in the literature1234567. The four main clinical features of onychomatricoma include thickening of the nail plate, transverse or longitudinal overcurvature, xanthonychia, and multiple splinter hemorrhage. After avulsion of the nail plate, onychomatricoma presents as a villous matrix tumor with projections that penetrate the thickened nail plate. Diagnosis is based on clinical, sonographic, and histopathological findings. The treatment for onychomatricoma is surgical, and the tumor must be removed completely. This is the second case of onychomatricoma in South Korea, which was initially misdiagnosed as onychomycosis in this patient. This is also the first reported case for which immunohistochemical staining of an onychomatricoma tissue sample was performed8.
In this report, we present clinical, histological, immunohistochemical, and sonographic findings of onychomatricoma in a 27-year-old Korean woman.
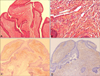
A 27-year-old woman presented with an 8-year history of gradual nail dystrophy on the right index finger, which was initially misdiagnosed as onychomycosis; she had been treated for 4 years with antifungal agents by general physicians without improvement. On physical examination, the nail plate of the right index finger was thickened and dark yellowish in color, mimicking onychomycosis. It exhibited scattered splinter hemorrhages and overcurvature in the longitudinal plane (Fig. 1). She had neither a history of trauma to the nail nor a personal or family history of skin cancer or dermatological disorders. A fungal culture of the nail plate yielded negative results, and radiography of the affected finger revealed no bone involvement linked to onychomatricoma. Ultrasonography revealed a hypoechogenic tumoral lesion in the nail matrix and a hyperechogenic area corresponding to fingerlike projections with low blood flow (Fig. 2). The nail was surgically removed, and the tumor at the nail matrix was excised. When the nail plate was initially avulsed, a firmly attached, filamentous tumor was observed arising from the nail matrix (Fig. 3A). The excision specimen was a flesh-colored tumor with fingerlike projections emerging from the nail matrix, leaving cavities in the nail plate (Fig. 3B). The proximal portion of the nail plate contained multiple cavities, which is characteristic of onychomatricoma (Fig. 3C). The normal nail plate continued to grow in 2 months after the excision (Fig. 3D). The excised tumor consisted of a connective tissue core and characteristic filiform projections lined by epithelium from the nail matrix lacking granular and horny layers (Fig. 4A, B). Immunohistochemical examination revealed that CD10 and CD34 were diffusely expressed in the stroma of the onychomatricoma (Fig. 4C, D). Periodic acid-Schiff histochemical stain was negative for fungal organisms.
Onychomatricoma, which is a benign tumor of nail matrix origin, has been rarely reported1234567. This may be partly due to the anatomic particularities of the nail apparatus and the often fragmented tissue specimens submitted to pathologists4. We report the clinical, radiological, sonographic, and histological features and treatment of a case of onychomatricoma, including a review of relevant literature.
Recent studies have reported that onychomatricoma mainly affects middle-aged women, with peak incidence at around the fifth decade of life23, although few case reports indicate that the onychomatricoma development has no sex predilection6. Onychomatricoma predominantly affects the fingers, with either single or multiple digits affected simultaneously1. Onychomatricoma may be complicated by fungal infection, and the tumor is often misdiagnosed as onychomycosis and treated as such910. In recent studies, patients with onychomatricoma considered to have genetic alterations, such as loss of chromosome11. The classic tetrad signs include yellowish, thickened nail plate, splinter hemorrhage, transverse overcurvature of the nail plate, and woodworm-like cavities in the distal margin of the nail plate. After avulsion of the nail plate, the presence of digitiform projections is quite suggestive of the diagnosis. A villous matrix tumor with projections penetrates the thickened nail plate.
Diagnosis is based not only on the classic tetrad signs but also on additional diagnostic methods, such as dermoscopy, ultrasonography, and histopathological findings. Dermoscopy shows perforations in the distal portion of the nail plate, hemorrhagic striae, and white longitudinal grooves corresponding to the nail plate channels12. Radiological examination shows no underlying bone involvement linked to onychomatricoma13. Ultrasonographic examination shows a hypoechoic tumoral lesion affecting the nail matrix and a hyperechogenic area corresponding to the fingerlike projections, in addition to having low blood flow14.
Onychomatricoma has distinctive histological features that confirm the diagnosis. Onychomatricoma is a fibroepithelial tumor comprising two distinct areas15. The proximal zone is located below the posterior nail fold and is characterized by deep epithelial invaginations occupied by overlying ungual protrusions. The distal zone, which corresponds to the lunula, comprises epithelial digitations originating from the matrix epithelium, which proliferate and cause perforations in the nail plate. Recently, Lee8 suggested that onychomatricoma might derive from the onychodermis because CD10, a marker of the onychodermis, is expressed in the stroma of onychomatricoma. Consistent with this finding, CD10 was diffusely expressed in the stroma of the onychomatricoma in our case. In addition, CD34, which is expressed in dendritic or fibroblast-like mesenchymal cells that are ubiquitously distributed in the dermis16, was diffusely expressed in the stroma of the onychomatricoma.
Misciali et al.17 reported onychoblastoma as a variant of onychomatricoma. Clinical examination showed a thickened nail plate with multiple cavities and a digitated tumor originating from the nail matrix, which is similar to that found for onychomatricoma. The tumor was distinguished from onychomatricoma by a unique arrangement with follicular differentiation and expression of cytokeratin (CK)5/6 and CK14 by keratin immunohistochemistry. However, Baran18 suggested that there was lack of evidence for differentiating onychoblastoma from onychomatricoma, and the gross morphology of this tumor and the channels within the nail plate were all consistent with onychomatricoma.
Differential diagnoses include fibrokeratoma, periungual fibroma, onychomycosis, squamous cell carcinoma, Bowen's disease, subungual verruca vulgaris, longitudinal melanonychia, and osteochondroma2. Among these, fibrokeratoma and periungual fibroma are the main histological differential diagnoses. In the longitudinal sections of the onychomatricoma, the histopathological features are reminiscent of those of fibrokeratoma. However, that diagnosis may be excluded because fibrokeratoma lacks the multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricomas19. Moreover, although ungual fibroma also produces longitudinal grooving of the nail plate due to the compression in the nail matrix, the differential diagnosis of onychomatricoma is possible because of the lack of hyperplastic onychomatricial epithelium7.
The treatment for onychomatricoma is surgical excision20. After anesthesia, the nail plate is removed, permitting visualization of the matrix tumor projections. The tumor must be completely excised, including the normal nail matrix proximal to the lesion, in order to prevent local recurrence5. Only one local recurrence has been reported within a mean follow-up time of 20.1 months4. With the exception of this case, most previous cases had no local recurrence2. Nail dystrophy can occur depending on the preservation of the nail matrix during tumor excision2.
The diagnosis of onychomatricoma is often delayed. Patients' typical delay in seeking medical attention may be attributed to the slow growth and absence of pain in most cases3. In addition, many cases are misdiagnosed as onychomycosis20, like our case, probably because dermatologists are not familiar with its clinical features. Therefore, we are reporting a typical case of onychomatricoma to help dermatologists recognize the clinical features of onychomatricoma and detect this uncommon nail matrix tumor early.
Figures and Tables
Fig. 1
(A) Yellowish, thickened nail plate of the right index finger, with splinter hemorrhages and longitudinal bands mimicking onychomycosis. (B) Lateral view of the nail plate showing longitudinal overcurvature.
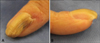
Fig. 2
Ultrasonographic image showing a 2×5×7 mm hypoechogenic tumor under the proximal nail fold (A, B) and hyperechogenic villous projections of the nail matrix having low blood flow (C). (A) Transversal view. (B, C) Longitudinal view.
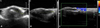
Fig. 3
(A) Intraoperative view of the filamentous tumor arising from the nail matrix. (B) Macroscopic appearance of the removed tumor with finger-like projections. (C) Proximal portion of the nail plate showing multiple cavities characteristic of onychomatricoma. (D) Normal nail plate growth 2 months after the excision.
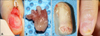
Fig. 4
(A, B) Histopathological section showing characteristic filiform projections lined by epithelium emerging from the nail matrix. (A) Medium-power view (H&E, ×100). (B) High-power view (H&E, ×400). (C) Immunohistochemical stain showing CD10 diffusely expressed in the stroma of the section. (D) Immunohistochemical stain showing CD34 diffusely expressed in the stroma of the section. (C, D) Medium-power view (×100).
ACKNOWLEDGMENT
This work was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2015R1C1A2A01055073).
References
1. Baran R, Kint A. Onychomatrixoma. Filamentous tufted tumour in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992; 126:510–515.

2. Tavares GT, Chiacchio NG, Chiacchio ND, Souza MV. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015; 90:265–267.

3. Di Chiacchio N, Tavares GT, Tosti A, Di Chiacchio NG, Di Santis E, Alvarenga L, et al. Onychomatricoma: epidemiological and clinical findings in a large series of 30 cases. Br J Dermatol. 2015; 173:1305–1307.

4. Perrin C, Baran R, Balaguer T, Chignon-Sicard B, Cannata GE, Petrella T, et al. Onychomatricoma: new clinical and histological features. A review of 19 tumors. Am J Dermatopathol. 2010; 32:1–8.

5. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009; 36:Suppl 1. 66–69.

6. Tosti A, Piraccini BM, Calderoni O, Fanti PA, Cameli N, Varotti E. Onychomatricoma: report of three cases, including the first recognized in a colored man. Eur J Dermatol. 2000; 10:604–606.
7. Baran R, Perrin C, Baudet J, Requena L. Clinical and histological patterns of dermatofibromas of the nail apparatus. Clin Exp Dermatol. 1994; 19:31–35.

8. Lee DY. The relation of onychomatricoma to onychodermis in the nail unit. Ann Dermatol. 2013; 25:394–395.

9. Piraccini BM, Antonucci A, Rech G, Starace M, Misciali C, Tosti A. Onychomatricoma: first description in a child. Pediatr Dermatol. 2007; 24:46–48.

10. Fayol J, Baran R, Perrin C, Labrousse F. Onychomatricoma with misleading features. Acta Derm Venereol. 2000; 80:370–372.

11. Cañueto J, Santos-Briz Á, García JL, Robledo C, Unamuno P. Onychomatricoma: genome-wide analyses of a rare nail matrix tumor. J Am Acad Dermatol. 2011; 64:573–578. 578.e1

12. Richert B, André J. Onychomatricoma. Ann Dermatol Venereol. 2011; 138:71–74.
13. Miteva M, de Farias DC, Zaiac M, Romanelli P, Tosti A. Nail clipping diagnosis of onychomatricoma. Arch Dermatol. 2011; 147:1117–1118.

14. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009; 145:1461–1462.

15. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998; 39:560–564.

16. Hisaoka M, Hashimoto H. Elastofibroma: clonal fibrous proliferation with predominant CD34-positive cells. Virchows Arch. 2006; 448:195–199.

17. Misciali C, Iorizzo M, Fanti PA, Piraccini BM, Ceccarelli C, Santini D, et al. Onychoblastoma (hamartoma of the nail unit): a new entity? Br J Dermatol. 2005; 152:1077–1078.

19. Yasuki Y. Acquired periungual fibrokeratoma--a proposal for classification of periungual fibrous lesions. J Dermatol. 1985; 12:349–356.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download