Abstract
Melkersson–Rosenthal syndrome (MRS) is an uncommon granulomatous disease characterized by the triad of relapsing facial paralysis, orofacial swelling, and fissured tongue. Genital swelling in MRS is rarely reported. We presented the first case of complete MRS with genital swelling in a child. Biopsy examinations of both the child's lower lip and penis showed noncaseating granuloma and intralymphatic granuloma infiltration. No symptoms or signs of other systemic disease (Crohn's disease or sarcoidosis) were observed after 2 years of follow-up. Genetic screening for CARD15/NOD2 in this patient showed negative, which further confirmed the diagnosis of MRS. Eleven other cases of suspected complete or incomplete MRS with genitalia involved were reviewed. Our case emphasizes the specific clinical feature of MRS with genitalia involved, which was genetically different from Crohn's disease and could be an independent entity. Lymphatic obstruction is responsible for localized edema in MRS.
Melkersson–Rosenthal syndrome (MRS) is an uncommon disease with unknown etiology characterized by recurrent orofacial edema, relapsing facial paralysis, and fissured tongue. Noncaseating epithelioid granuloma was a main histopathologic feature12. In patients with MRS, other sites beyond the orofacial region are very rarely reported. Here, we presented one boy diagnosed with MRS with genital swelling, and reviewed related literatures.
A 12-year-old boy presented with relapsing swelling of the lower lip and penis for 2 years and right facial paralysis for 20 days. He had no respiratory or gastrointestinal problems and denied any history of allergy, irritants contact, local trauma, or infection. Physical examination showed localized swelling of the lower lip with slight crust and fissure on the surface (Fig. 1A), deep fissures in the dorsum of the tongue (Fig. 1B), and cobblestone-like hypertrophy of the buccal mucosa. A droop of mouth angle and a shallower nasolabial groove on his right face were noted (Fig. 1C). No oral ulceration or gingival hyperplasia was observed. His penis showed a circumscribed edematous patch with mild crusts (Fig. 1D). The results of the rest of his physical examination were unremarkable, and there was no similar record in his family.
Laboratory tests revealed slightly elevated lymphocyte counts (5.3×109/L) and immunoglobulin E level (114 IU/ml). Electromyography of the right peripheral facial nerve showed delayed motor conduction and decreased amplitude, suggesting nerve fibril damage. Results of other routine tests—including erythrocyte sedimentation rate, C-reactive protein, antistreptolysin O, rheumatoid factor, antinuclear antibody, serum immunoglobulin (IgA, IgM, IgG), complement components (C3, C4, and C1 inhibitor), allergen detection, and chest radiogram—were all either within normal ranges or negative.
Examination of skin biopsy specimens from the lower lip and penis showed identical histological changes. The epidermis showed mild hyperkeratosis, parakeratosis, and acanthosis with scattered vacuolar degeneration of basal cells and epidermotropic lymphocytes. Several well-formed noncaseating granulomas were distributed throughout the dermis, and most of them surrounded or filled into dilated vessels (Fig. 2). A large number of CD68-positive histiocytes were crowded in the granuloma region (Fig. 3A), and some of them were even located only within dilated vessels (Fig. 3B). Immunohistochemical D2-40 staining showed that most of the affected vessels were lymphatic vessels (Fig. 3C); blood vessels marked by CD31 were normal size; and no granulomas were detected in their cavities (Fig. 3D).
All 12 exons of the CARD15/NOD2 gene extracted from the patient's peripheral blood were also sequenced and analyzed, and only one single nucleotide polymorphism was found: rs1861759 (c.1761 T>G; R587R), which indicated a single nucleotide polymorphism mutation.
The boy was diagnosed with MRS and treated with oral methylprednisolone 8 mg per day and topical 0.03% tacrolimus cream. The two lesions improved after 3 months of therapy, and then oral methylprednisolone was tapered and stopped after 6 months. Topical tacrolimus cream and oral thymosin were used as a maintenance regimen since then. There was significant improvement on his lower lip (Fig. 1E) and penis (Fig. 1F), and no symptoms or signs of other systemic diseases (Crohn's disease [CD] or sarcoidosis) were observed during the 2 years of follow-up.
MRS is an uncommon granulomatous disease. The diagnosis of MRS is often difficult because the complete triad is reported in only 8% to 18% of MRS123, whereas granulomatous cheilitis without facial paralysis—also called incomplete MRS—is more common.
The most common manifestation of MRS is nonpitting swelling in the orofacial region, and the upper lip is the most frequently involved site; the lower lip is less frequently involved4. Intraoral signs include swelling of gingival and mucosa, fissured tongue, and ulceration234. Recurrent genital swelling in either complete or incomplete MRS is very rare; we found only 11 suspected cases reported in the literature (Table 1)56789101112131415. Genital swelling in MRS is similar to orofacial swelling in clinical features and pathological changes13. Patients generally presented with painless, circumscribed, and firm edema in the anoperineal region. It is worth noting that half of those cases were complicated with CD, and actually, whether MRS and CD belong to the same disease spectrum has long been controversial.
CARD15, originally named NOD2, was the first identified susceptibility gene of CD. Recent studies showed it was also associated with other systemic granulomatous diseases like Blau syndrome and early-onset sarcoidosis16. CARD15 was essential for innate immunity and control of inflammation, and when dysregulated, it caused inflammation disease via aberrant activation of NF-κB and mitogen- activated protein kinase pathways and played a pathogenic role in the formation of granulomas16. MRS shared many clinical and pathological similarities with other granulomatous diseases, whereas Gale et al.17 reported that MRS and CD were genetically different because none of the 17 Swedish patients with complete or incomplete carried any CD-linked CARD15 variation. In our case, we found just one single nucleotide polymorphism— c.1761 T>G (R587R)—which indicated that CARD15 was not involved in the pathogenesis of the patient. The boy had no gastrointestinal symptoms, his fecal occult blood test was repeatedly negative after 2 years of follow-up, and he had typical clinical triad symptoms and noncaseating granuloma of MRS; therefore, he was diagnosed with complete MRS with genitalia involved, which had never before been reported in a child.
MRS usually occurs in childhood and adolescence, and its exact etiology remains to be elucidated, although several risk factors, including infection, allergy, hereditary predisposition, autoimmune disorder, and foreign-body irritation, have been discussed in the literature123. The characteristic histopathological finding in MRS is noncaseating granuloma. The granuloma is distributed mainly in the superficial and middle layers of dermis and is composed of histiocytes, lymphocytes, and small numbers of multinucleated giant cells and plasma cells. The pathological mechanism of localized edema in MRS is not well understood. In the boy, we observed intralymphatic granuloma in two lesions, whereby we proposed that lymphatic vessel obstruction and destruction caused by infiltrating granuloma may contribute to localized edema of MRS, which agreed with the results of González-García et al.18.
There is no known satisfactory cure for MRS. Systemic or intralesional corticosteroid is often temporarily beneficial. Immunosuppressive regimens, like clofazimine, dapsone, sulphasalazine, thalidomide, and minocycline, have also been reported to be alternatives to glucocorticoid. Surgery is recommended for severe cases and for patients who cannot tolerate glucocorticoid treatment19.
By presenting our case and reviewing reported MRS with genitalia involvement, we demonstrated that genital swelling as a special complication of MRS should be noticed and that lymphatic obstruction possibly contributes to localized edema.
Figures and Tables
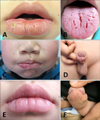 | Fig. 1Clinical images of the patient. Erythematous swelling of the lower lip with crust and fissure on the surface (A), fissured tongue (B), droop of the mouth angle, shallower nasolabial groove on the right face (C), edematous swelling of the penis (D), significant improvement of the lower lip (E), and penis (F) after 2 years' therapy. |
 | Fig. 2Hematoxylin-eosin staining results. (A) Biopsy examination of the lower lip showed lymphocytes, histiocytes, and plasma cells accumulated in the dermis, forming noncaseating granuloma (×100). (B) Biopsy examination of the penis. Noncaseating granuloma can also be observed in the biopsy examination of the penis (×200). (C) Biopsy specimen of the lower lip. Some granuloma is located in the deep muscle layer (×400). (D) Biopsy specimen of the penis. Histiocytes were found within dilated vessels ×400). |
 | Fig. 3Immunohistochemical staining results. (A) Histiocytes marked by CD68 were crowded in the granuloma region (×100). (B) Some CD68-positive histiocytes are located within dilated vessel (×400). (C) Histiocytes and lymphocytes can be observed within the lymphatic vessels highlighted by D2-40 in and outside the granuloma region (×200). (D) Blood vessels marked by CD31 can also be found in the center of granuloma with empty cavities (×400). |
Table 1
Complete or incomplete MRS accompanied by anogenital swelling reported in literature

| No. | Sex/Age (yr) | Clinical manifestations | Treatment | First author (year) | Reference |
|---|---|---|---|---|---|
| 1 | F/40 | Swelling of face and vulva | Steroids and clofazimin | Knopf (1992) | 5 |
| 2 | F/19 | Swelling of vulva and upper lip for 2 years and diagnosed as complete MRS | Oral prednisone | Lloyd (1994) | 6 |
| 3 | F/32 | Swelling of labia for 9 yearsswelling of lower lip 2 years later | Surgical resection | Guerrieri (1995) | 7 |
| 4 | F/30 | Swelling of genitalia and lip for 18 months | Mercaptopurine, clofazimin, morphine | Ilnyckyj (1999) | 8 |
| 5 | F/36 | Swelling of genital for 3 years swelling of upper lip for 6 months | Metronidazole | Sbano (2007) | 9 |
| 6 | F/8 | Swelling of genitalia, left cheek, and lips for 8 months | Oral prednisone | Nabatian (2011) | 10 |
| 7 | F/37 | Bilateral vulval lumps for 2 years, with history of lip swelling 4 years before | Intravenous infliximab | Wickramasinghe (2012) | 11 |
| 8 | M/46 | Swelling of lips for 11 years swelling of penis for 6 years | Prophylactic penicillin | Makatsori (2013) | 12 |
| 9 | F/30 | Swelling of upper lip for 10 years swelling of lower lip and vulva for 6 months | Glucocorticoids, antibiotics | Kambil (2013) | 13 |
| 10 | M/21 | Swelling of penis for 6 weeks, with history of orofacial granulomatosis 2 years before | Antibiotics, oral prednisolone | Gordon (2013) | 14 |
| 11 | M/13 | Swelling of scrotum for 6 days, withhistory of upper lip swelling 2 years before | Antitumor necrosis factor alpha | Rajah (2014) | 15 |
| 12 | M/12 | Swelling of lower lip and penis for 2 years transient right facial paralysis | Oral methylprednisolone, topical tacrolimus cream | Zhaowei Chu (2016) | Present study |
ACKNOWLEDGMENT
This work was supported by the Program for New Century Excellent Talents in University (China) (NCET-10-0673).
References
1. Elias MK, Mateen FJ, Weiler CR. The Melkersson-Rosenthal syndrome: a retrospective study of biopsied cases. J Neurol. 2013; 260:138–143.

2. Ziem PE, Pfrommer C, Goerdt S, Orfanos CE, Blume-Peytavi U. Melkersson-Rosenthal syndrome in childhood: a challenge in differential diagnosis and treatment. Br J Dermatol. 2000; 143:860–863.

3. Liu R, Yu S. Melkersson-Rosenthal syndrome: a review of seven patients. J Clin Neurosci. 2013; 20:993–995.

4. Dodi I, Verri R, Brevi B, Bonetti L, Balestrier A, Saracino A, et al. A monosymptomatic Melkersson-Rosenthal syndrome in an 8-year old boy. Acta Biomed. 2006; 77:20–23.
5. Knopf B, Schaarschmidt H, Wollina U. Monosymptomatic Melkersson-Rosenthal syndrome with subsequent vulvitis and perivulvitis granulomatosa. Hautarzt. 1992; 43:711–713.
6. Lloyd DA, Payton KB, Guenther L, Frydman W. Melkersson-Rosenthal syndrome and Crohn's disease: one disease or two? Report of a case and discussion of the literature. J Clin Gastroenterol. 1994; 18:213–217.
7. Guerrieri C, Ohlsson E, Rydén G, Westermark P. Vulvitis granulomatosa: a cryptogenic chronic inflammatory hypertrophy of vulvar labia related to cheilitis granulomatosa and Crohn's disease. Int J Gynecol Pathol. 1995; 14:352–359.
8. Ilnyckyj A, Aldor TA, Warrington R, Bernstein CN. Crohn's disease and the Melkersson-Rosenthal syndrome. Can J Gastroenterol. 1999; 13:152–154.

9. Sbano P, Rubegni P, Risulo M, De Nisi MC, Fimiani M. A case of idiopathic granulomatous cheilitis and vulvitis. Int J Dermatol. 2007; 46:720–721.

10. Nabatian AS, Shah KN, Iofel E, Rosenberg S, Javidian P, Pappert A, et al. Asymptomatic granulomatous vulvitis and granulomatous cheilitis in childhood: the need for Crohn disease workup. J Pediatr Gastroenterol Nutr. 2011; 53:100–101.

11. Wickramasinghe N, Gunasekara CN, Fernando WS, Hewavisenthi J, de Silva HJ. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn's disease: dramatic response to infliximab therapy. Int J Dermatol. 2012; 51:966–968.

12. Makatsori M, Manson AL, Gurugamai P, Wakelin S, Seneviratne SL. Penile granulomatosis presenting as pseudoangioedema. Eur Ann Allergy Clin Immunol. 2013; 45:111–112.
13. Kambil SM, Bhat RM, Dandakeri S, Bisen N. Granulomatous cheilitis with granulomatous vulvitis: a rare association. Indian J Dermatol Venereol Leprol. 2013; 79:799–801.

14. Gordon KD, Brice G, Walker Y, Pollok R, Mortimer P, Slater C. Genital lymphoedema due to ano-genital granulomatosis. Int J STD AIDS. 2013; 24:149–151.

15. Rajah K, Oliver MR, McLeod L, Orchard D, Leal M. Unusual manifestations of a common gastrointestinal disorder. J Paediatr Child Health. 2014; 50:158–160.

16. Borzutzky A, Fried A, Chou J, Bonilla FA, Kim S, Dedeoglu F. NOD2-associated diseases: Bridging innate immunity and autoinflammation. Clin Immunol. 2010; 134:251–261.

17. Gale G, Östman S, Rekabdar E, Torinsson Naluai Å, Högkil K, Hasséus B, et al. Characterisation of a Swedish cohort with orofacial granulomatosis with or without Crohn's disease. Oral Dis. 2015; 21:e98–e104.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download