Dear Editor:
Eruptive melanocytic lesions (EMLs) have been associated with immunosuppressive conditions, immunodeficiency, and blistering diseases. Melanocytic lesions that suddenly develop after the administration of immunosuppressants indicate that the immune system may play an important role in limiting normal melanocyte proliferation1. The development of EML is probably mediated by the immunosuppressive action of drugs2.
Biological drugs such as infliximab, alefacept, etanercept, and sorafenib have also been reported to be associated with EML134. We report a case of EML and a dysplastic nevus that developed in a patient with chronic myeloid leukemia (CML) after using nilotinib.
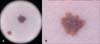
A 39-year-old female patient was found to have CML after a routine health evaluation and began to take 400 mg nilotinib twice a day from 2008; she was still taking the medication at the time of the study. After several months, she gradually developed pigmented lesions on her face and trunk. During the next 4 years, the number of pigmented lesions increased, appearing throughout the whole body, including areas that are not exposed to sunlight. On examination, we found ≥100 pigmented lesions with diameters of 2~3 mm. In addition, 4 years after the initiation of therapy, she noticed a 4-mm dark papule on her right thigh, with itching. Dermoscopic examination of the papule on the thigh showed structureless pigmentation with asymmetric and irregular and fuzzy borders, and several blackish globules were found on one side of the periphery (Fig. 1A, B). Histopathological examination of the 3-mm, benign melanocytic lesion showed slightly elongated, clubbed rete ridges and intermittently distributed single melanocytes at the tip and side of the rete ridges without signs of dysplasia (Fig. 2A). In contrast, the atypical pigmented lesion showed elongated, fused rete ridges and contiguously distributed single melanocytes and a few small nests at the dermal-epidermal junction. In addition, eosinophilic fibroplasias and mild perivascular infiltrate were observed. The lesional melanocytes were observed to have moderately enlarged nuclei and slightly hyperchromatic nucleoli (Fig. 2B~D). Therefore, we established a diagnosis of dysplastic nevus with EML that developed during nilotinib therapy. We removed the dysplastic nevus through punch biopsy and the multiple melanocytic lesions with a 755-mm alexandrite laser.
Nilotinib is a second-generation tyrosine kinase inhibitor with excellent efficacy for CML5. The effects of nilotinib as a KIT inhibitor were also reported to show a durable response in patients with metastatic melanoma harboring the KIT mutation5. However, there have been no clinical or laboratory reports of nilotinib affecting the proliferation of melanocytes or tumor development from a melanocyte origin. Nevertheless, the development of EML after nilotinib use may possibly be caused by interference with the functions that control the proliferation of melanocytes through similar mechanisms as those of sorafenib-3 or tumor necrosis factor-α inhibitor-induced EML14. Because the development of eruptive pigmented lesions has been related to immunosuppression, potentially diminished immune surveillance may allow melanocyte growth factors to the promote development of pigmented lesions3.
To our knowledge, this is the first reported case of a dysplastic nevus with EML that developed during nilotinib therapy in a middle-aged woman with CML.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download