Abstract
Venous leg ulcers, the most common form of leg ulcers, are relevant to the pathogenicity of pericapillary fibrin cuff. Sarcoidosis, a multiorgan granulomatous disease, causes fibrin deposition in tissues. We report a case of a 50-year-old man with venous leg ulcers coexisting with sarcoidosis. On the basis of the histologic findings, we propose the hypothesis that sarcoidosis patients are prone to the development of venous leg ulcers.
The risk factors for venous leg ulcers include old age, obesity, varicose veins, a history of significant leg injury, phlebitis, and heart disease1. Here, we describe an aged Korean male patient with venous leg ulcers and hyperpigmentation on both lower legs, who was consequently found to have sarcoidosis. We consider that this patient may provide a basis for an association between venous leg ulcers and sarcoidosis.
A 50-year-old man presented with painful ulcers and hyperpigmentation on both lower legs (Fig. 1A). He also showed extensive annular pigmentary skin lesions on the chest and upper back. The lesions began to develop several years ago and had deteriorated recently, within the last year. Ulcers were found on both ankles, as well as the lower third part of both lower legs, with yellowish material, accompanied by mild lower-leg pitting edema. In addition, he complained of multiple erythematous macules with ulcer and crust on the torso, accompanied by pruritus (Fig. 1B).
The pulse of the dorsalis pedis artery was well palpable on physical examination, and the ankle-brachial index was measured as 1.18, which is within the reference range. There was no specific finding in the laboratory examinations, except for an elevated plasma fibrinogen level of up to 516 (reference range, 170~350). To exclude pulmonary vein thrombosis and deep vein thrombosis, chest computed tomography and leg magnetic resonance imaging were performed. They showed multiple bronchial lymph node enlargements instead of deep vein thrombosis and atherosclerosis. Skin biopsies were performed in the lower leg (Fig. 2) and chest (Fig. 3). In the histopathologic examination of the leg specimen, fibrin material overlying the epidermis, dermal fibrosis, perivascular lymphocytic infiltration, and extravasation of red blood cells were observed. In the histopathologic examination of the chest specimen, no specific finding other than inflammatory skin reactions, like perivascular lymphocytic infiltration without evidence of granulomatous inflammation, was noted. A lymph node biopsy was obtained through endobronchial ultrasound-guided transbronchial needle aspiration, which showed chronic granulomatous inflammation with multinucleated giant cells. The result of the interferon- gamma release assay was negative. There was a decreased diffusing capacity of the lungs for carbon monoxide of 68%, representing a restrictive pattern in the lung function test.
On the basis of these findings, sarcoidosis was diagnosed. He was referred to the department of rheumatology and prescribed with 4 mg methylprednisolone every other day for the treatment of sarcoidosis. His cutaneous lesions on the torso and lower legs were diagnosed as nonspecific lesions of cutaneous sarcoidosis and venous leg ulcers, respectively. Pentoxifylline (400 mg) and folic acid (1 mg) were administered on a daily basis, in addition to methylprednisolone. After 5 weeks, the lower-leg ulcers were healed, leaving hyperpigmentation. Moreover, the skin lesions on the torso were much improved, with decreased associated symptoms such as pruritus.
Venous leg ulcers account for more than half of all chronic leg ulcers2. It is known that there are several risk factors for venous leg ulcers1. However, the present patient did have not any of the risk factors for venous leg ulcers, such as obesity, old age, smoking, deep venous thrombosis, history of significant leg injury, diabetes, hypertension, and hypercholesterolemia. The diagnosis of sarcoidosis was the only specific factor.
To date, it is known that chronic venous insufficiency is responsible for the development of venous ulceration. However, there is no agreement on the pathogenic mechanism. Burnand et al.3 and Pardes et al.4 suggested a hypothesis that pericapillary fibrin cuff impedes oxygen and nutrient diffusion, which leads to a dermal microenvironmental change that induces cell damage and delayed wound healing. In addition, chronic venous insufficiency causes distension of the vessel wall and hemoconcentration in the capillary5, which makes the vascular structure more permeable. Consequently, macromolecules, such as fibrinogen, leak into the extravascular space, forming pericapillary fibrin cuffs.
Falanga et al.6 and Falanga7 found increased plasma total fibrin-related antigen and D-dimer level in patients with venous disease, reflecting elevated fibrin formation in the body. In addition, it was observed that fibrin cuff encircles the dermal capillary of the venous ulcer lesion on direct immunofluorescence.
Sarcoidosis is a multiorgan granulomatous disease with unknown etiology and with variable skin manifestations89. Especially, leg swelling has been described as a nonspecific symptom of sarcodiosis10, which means that a patient with sarcoidosis would have chronic venous insufficiency. An immunopathological study has revealed that a fibrin network was observed within the granulomas11. D-dimer, a fibrin degradation product, is elevated in the bronchoalveolar lavage fluid and plasma in patients with pulmonary sarcoidosis12. Moreover, its level is associated with disease activity13.
Overall, both venous leg ulcers and sarcoidosis have common histologic manifestations and biochemical findings, with respect to fibrin deposition in the tissue and elevated D-dimer level in plasma. Furthermore, they share some pathophysiologic processes in terms of chronic venous insufficiency.
On the basis of these findings, it may be hypothesized that sarcoidosis could manifest as chronic venous insufficiency, which causes venous leg ulcer. Moreover, the greater the severity of sarcoidosis, which indicates a higher level of fibrin in plasma, the more accelerated the formation of fibrin cuffs and venous leg ulcers will be (Fig. 4). To the best of our knowledge, the association between sarcoidosis and venous leg ulcers has never been previously reported. In this report, we present the hypothesis that sarcoidosis patients are at a higher risk to develop venous leg ulcers, on the basis of the clinical manifestation and the common pathology between the two different diseases. To determine this correlation, further molecular and epidemiologic studies with a large population are needed.
Figures and Tables
Fig. 1
(A) Painful ulcers with yellowish material and hyperpigmentation on both lower legs, especially on both ankles. (B) Multiple erythematous macules with ulcer and crust on the torso.
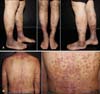
Fig. 2
(A) Thick eosinophilic material layer overlying the spongiotic epidermis. (B) Dermal fibrosis and perivascular lymphocytic infiltration. (C) Extravasation of red blood cells with lymphocytes in the dermis. A~C: H&E.

References
1. Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case-control study. J Vasc Surg. 1995; 22:622–628.

2. Nelzén O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg. 1994; 81:182–187.

3. Burnand KG, Whimster I, Naidoo A, Browse NL. Pericapillary fibrin in the ulcer-bearing skin of the leg: the cause of lipodermatosclerosis and venous ulceration. Br Med J (Clin Res Ed). 1982; 285:1071–1072.

4. Pardes JD, Tonneson MG, Falanga V, Eaglstein WH, Clark RAF. Skin capillaries surrounding chronic venous ulcers demonstrate smooth muscle hyperplasia and increased laminin type IV collagen. J Invest Dermatol. 1990; 94:563.
5. Moyses C, Cederholm-Williams SA, Michel CC. Haemoconcentration and accumulation of white cells in the feet during venous stasis. Int J Microcirc Clin Exp. 1987; 5:311–320.
6. Falanga V, Kruskal J, Franks JJ. Fibrin- and fibrinogen-related antigens in patients with venous disease and venous ulceration. Arch Dermatol. 1991; 127:75–78.

8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999; 160:736–755.
9. Kang MJ, Kim HS, Kim HO, Park YM. Cutaneous sarcoidosis presenting as multiple erythematous macules and patches. Ann Dermatol. 2009; 21:168–170.

10. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill Professional;2012. p. 1869–1880.
11. Quismorio FP Jr, Sharma OP, Chandor S. Immunopathological studies on the cutaneous lesions in sarcoidosis. Br J Dermatol. 1977; 97:635–642.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download