Abstract
Nodular vasculitis was introduced by Montgomery for cases of erythema induratum-like lesions that were not associated with tuberculosis. Nodular vasculitis has been associated with both nontuberculous infections and noninfectious conditions. However, there has been no report on the development of nodular vasculitis during tumor necrosis factor-α inhibitor treatment. A 28-year-old man visited our clinic for the treatment of severe psoriasis with a 20-year history. Subcutaneous injection of etanercept (25 mg, twice weekly) was started. One year later, erythematous nodules developed on his lower leg. A skin biopsy showed lobular panniculitis with extensive necrosis and vasculitis. To exclude latent tuberculosis, an assay specific for Mycobacterium tuberculosis antigens was performed, with a negative result. After stopping etanercept under the diagnosis of nodular vasculitis associated with etanercept, the lesions gradually disappeared, leaving depressed scars in 3 months. There has been no recurrence after 6 months of follow-up.
In 1945, nodular vasculitis was introduced by Montgomery1 for cases of erythema induratum-like lesions that were not associated with tuberculosis. Nodular vasculitis has been associated with various factors. Infectious causes have been reported, such as Nocardia, Pseudomonas, Fusarium, and Chlamydia23. It may also be associated with hepatitis B virus and hepatitis C virus45. Noninfectious conditions may include superficial thrombophlebitis, hypothyroidism, chronic lymphocytic leukemia, rheumatoid arthritis, Crohn's disease, and systemic lupus erythematosus46. Drug-induced nodular vasculitis has been rarely reported until now, and one case has been described in relation to propylthiouracil7. To our knowledge, there has been no report of nodular vasculitis associated with tumor necrosis factor (TNF)-α inhibitor in the English literature. Therefore, here, we discuss the relation between etanercept and nodular vasculitis.
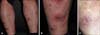
A 28-year-old man visited our dermatologic clinic with a 20-year history of psoriasis. He had been treated with retinoid (acitretin) and phototherapy (narrow-band ultraviolet B). Because he did not respond to the treatments and the skin lesions persisted, etanercept (25 mg, twice weekly) had been tried with a partial improvement (reduction of psoriasis area and severity index score from 23.4 to 14.7). One year later, erythematous painful nodules developed on the lower legs. On clinical examination, there were erythematous plaques with scale on the whole body, and multiple erythematous tender nodules with central crust was found on both of his lower legs (Fig. 1A, B). Laboratory examination revealed normal liver and kidney functions, and a normal urine sediment. Serology for hepatitis B and C and human immunodeficiency virus infection were negative. Screening for antinuclear antibody, anti-neutrophil cytoplasmic antibodies, anti-DNA antibody, and anti-lupus anticoagulant was negative, and complements 3 and 4 were normal. Interferon-gamma release assays specific for Mycobacterium tuberculosis antigens was performed to exclude latent tuberculosis, and the result was negative. Histopathologic examination of the cutaneous lesion revealed a septal and lobular panniculitis with extended necrosis and leukocytoclastic vasculitis (Fig. 2). On the basis of the clinical, histologic and laboratory findings, nodular vasculitis that might have been associated with etanercept was diagnosed. Subsequently, we started to treat him with 10 mg prednisolone with discontinuation of etanercept. The prednisolone treatment was stopped as he complained of diarrhea and abdominal pain following the treatment. His skin lesion gradually improved without any medications, and left depressed scars after 3 months (Fig. 1C). There was no relapse up to 6 months after the resolution.
TNF-α inhibitors are effective for the management of several systemic autoimmune diseases such as rheumatoid arthritis, Crohn's disease, and psoriasis8. However, increasing anti-TNF-α therapies caused the development of secondary autoimmune diseases, such as cutaneous vasculitis, lupus-like syndrome, and interstitial lung disease, paradoxically induced by anti-TNF-α agents8. Cutaneous vasculitis associated with TNF-α inhibitor has been described with various clinical manifestations, including purpura, blisters, erythematous macules, and ulceration9. This case initially presented an erythematous tender nodule with a central crust and left a depressed scar. In addition to the results of physical examination, the histologic manifestations were compatible to nodular vasculitis. Although the development of nodular vasculitis during TNF-α inhibitor treatment has not been reported thus far, it is possible that nodular vasculitis might have been associated with etanercept because nodular vasculitis is a rare variant of cutaneous vasculitis that shares the pathogenesis of vasculitis associated with the TNF-α inhibitor. However, the exact mechanism of the development of nodular vasculitis during TNF-α inhibitor treatment remains unknown. Several hypotheses have been suggested. First, the development of antibodies against the TNF-α inhibitor might induce cutaneous vasculitis10. Second, inhibition of TNF-α increases type 1 interferon by changing the balance between Th1 and Th2 cytokines, which may cause the development of autoimmune diseases such as dermatomyositis, systemic lupus erythematosus, and vasculitis.
We considered that this case of nodular vasculitis was induced by etanercept11. However, it may not be understood from several points. First, it occurred long (1 year) after the initiation of treatment. The mean anti-TNF-α therapy duration was 9.5 months in the study by Ramos-Casals et al.12 This result, vasculitis due to anti-TNF-α agents, revealed a slower time of onset than that in drug rash. Second, it might have been generated by asymptomatic infection. Nodular vasculitis associated with Chlamydia infections, which can present as an asymptomatic infection, has been reported and complete elimination is difficult3. Last, there is a possibility that vasculitis was caused by psoriasis. The relation between psoriasis and vasculitis is still under debate. Some papers have reported on its association; however, unlike this case, many more cases were those of pustular psoriasis13. Although we could not completely solve these doubtful questions, the patient had plaque-type psoriasis and did not have a drug history, infectious symptoms, and signs. Moreover, his skin lesions showed improvement after discontinuing etanercept, and they did not recur for 6 months. In this regard, we believe that there is a high possibility that the disease, nodular vasculitis, had been caused by etanercept. Therefore, we report the possibility of nodular vasculitis occurring in association with etanercept.
Figures and Tables
References
1. Montgomery RM. A case for diagnosis; dermatophytosis; nodular vasculitis? Arch Derm Syphilol. 1948; 57:580.
2. Patterson JW, Brown PC, Broecker AH. Infection-induced panniculitis. J Cutan Pathol. 1989; 16:183–193.

3. Sakuma H, Niiyama S, Amoh Y, Katsuoka K. Chlamydophila pneumoniae infection induced nodular vasculitis. Case Rep Dermatol. 2011; 3:263–267.

4. Segura S, Pujol RM, Trindade F, Requena L. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008; 59:839–851.

5. Ural I, Erel A, Ozenirler S, Tekin NS, Gurert MA. Nodular vasculitis associated with chronic hepatitis C. J Eur Acad Dermatol Venereol. 2002; 16:298–299.

6. Westers-Attema A, van Tubergen A, Plasschaert H, van Marion AM, Frank J, Poblete-Gutiérrez P. Nodular vasculitis in systemic lupus erythematosus. Int J Dermatol. 2008; 47:Suppl 1. 3–6.

7. Wolf D, Ben-Yehuda A, Okon E, Naparstek Y. Nodular vasculitis associated with propylthiouracil therapy. Cutis. 1992; 49:253–255.
8. Kerensky TA, Gottlieb AB, Yaniv S, Au SC. Etanercept: efficacy and safety for approved indications. Expert Opin Drug Saf. 2012; 11:121–139.

9. Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin Proc. 2012; 87:739–745.

10. Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009; 61:486–504.

12. Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007; 86:242–251.
13. Wong SS, Marks R. Cutaneous vasculitis in psoriasis. Acta Derm Venereol. 1994; 74:57–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download