Abstract
Background
In the treatment of keloids, the recurrence after surgical excision is relatively high. Various types of adjuvant therapy such as radiotherapy and corticosteroid injection have been used to reduce the recurrence.
Objective
The aim of this study was to determine the appropriate time for initiating postoperative radiotherapy and to analyze factors associated with the occurrence and recurrence of keloids.
Methods
Of these 37 lesions, 22 were located in the ear lobe, 6 in the helix of the auricle, 4 on the shoulder, 3 on the chest wall, and 2 on the abdomen. Causative factors were piercings (n=24), trauma (n=5), previous surgical lesions or bacillus Calmette-Guerin vaccination lesions (n=3) and acne (n=2). Radiation therapy was initiated within 24 h in 24 lesions, between 24 and 72 h in 6 lesions, and after more than 72 h in 7 lesions.
Results
Seven lesions recurred, including 5 recurrences in high stretch-tension regions (p=0.010). Initial treatments were administered within 24 h in 1 lesion and more than 72 h after surgical excision in 6 lesions (p<0.0001). In the 19 patients with family histories, maternal and paternal genetic predispositions were present in 14 and 5 patients, respectively (p=0.033).
Conclusion
Radiotherapy should be initiated within 72 h of surgical excision. Location in a high stretch-tension region was significantly associated with recurrence. Patients with a family history showed a significant tendency toward maternal genetic predisposition. Therefore, combination therapy should be considered to reduce the occurrence and recurrence of keloids, and careful observation is required.
Keloids are defined as dense fibrous tissue arising from an abnormal healing response to a cutaneous injury that grows beyond the boundaries of the original wound scar1,2,3. Keloids, which are characterized by a collection of atypical fibroblast with excessive deposition of extracellular matrix components such as collagen, fibronectin, elastin, and proteoglycans, can be considered a "benign neoplasm that does not know when to stop"1. Pathologically, keloids contain relatively acellular centers and thick, abundant collagen bundles that, form nodules in the deep dermal portion of the lesion. Keloids can occur on any part of the body, but they typically, occur on the ear lobes, shoulders, chest, and back2,3. Many unknown factors can cause keloids. However, commonly kwon causative factors of keloids are piercings, trauma, surgical scars, acne scars, and chickenpox scars1,3. The incidence of keloids is in both sexes, although a female predominance has been reported, most likely due to higher frequency of ear lobe piercings in women2,3,4. When they occur in the head and neck area, keloids are associated with unpleasant cosmetic consequences, especially in younger patients1,2,3,4. In addition to, cosmetic problems, keloid patients can experience itching, redness, and unusual sensations2,3. Although they are more likely to develop in people with darker skin, keloids can occur all skin types. The tendency to form keloids sometimes appears to run in families. Notably, the connection between family history and a hereditary tendency for keloid formation has been controversial; some authors have reported that 10% of patients may have a family history, but no specific research on family history has been reported5,6. Various methods for the treatment of keloids have been attempted, including intralesional steroids, interferon and fluorouracil injections, laser therapy, cryotherapy, silicone gel sheets applications, surgical excision, external radiation therapy, and brachytherapy1,4. However, these treatments are largely in effective, and the lesions often return. Moreover, despite the many treatment methods attempted, a satisfactory method has yet to be reported. Many authors have reported on the surgical excision of keloids, which is followed by recurrence in 50%~80% of patients1,7. Therefore, adjuvant treatment such as radiation therapy has also been considered as a means of improving therapeutic outcomes after surgery1,7,8. A various of radiation techniques such as electron beam radiotherapy, and iridium-192 brachytherapy with implants or strontium-90 dermal plates can be used, and postoperative radiotherapy is a highly effective therapeutic method7,8. This study aimed to evaluate the results of postoperative electron beam radiotherapy, and to analyze factors associated with keloid occurrence and recurrence.
From January 2006 to January 2012, 30 patients with 37 keloid lesions were treated with postoperative electron beam radiotherapy. There were 23 female patients (29 lesions) and 7 male patients (8 lesions) with, ages ranging from 11 to 66 year (mean, 23.9 years). Sites of keloid development included the ear lobe (n=22, 59.5%), the helix of the auricle (n=6, 16.2%), the shoulder (n=4, 10.8%), the chest wall (n=3, 8.1%) and the abdomen (n=2, 5.4%). All lesions were surgically excised, and the clinical diagnosis of keloids was confirmed histopathologically. Wide local excision was performed for 21 lesions, and core excision was performed for 16 lesions. Factors contributing to keloid formation included piercing (24 lesions; 64.9%), trauma (5 lesions; 13.5%), surgical scars (3 lesions; 8.1%), bacillus Calmette-Guerin vaccination scars (3 lesions; 8.1%), and acne (2 lesions; 5.4%). A total of 19 patients (22 lesions) had a family history. Among these, maternal and paternal hereditary tendencies were present in 14 patients (16 lesions, 73.7%), and 5 patients (6 lesions, 26.3%), respectively. Because more than 50% of keloid recurrences occur within 6 months of treatment3,5,7, a follow-up period of at least 6 months was necessary and we observed the treated lesions every 3 months after radiotherapy. The median follow-up period was 27.4 months (range, 9~51 months) (Table 1). Our study protocol was approved by the institutional review board of Chonbuk National University Hospital (CUH 2012-02-003-001).
Radiation therapy was applied with a 6-MeV electron beam. To ensure an adequate radiation dose was applied to the surface of the skin, a 0.5 or 1-cm thick silicone bolus such as a tissue compensator was placed on the keloid surgical scar, based on the characteristics of the scar. The electron beam irradiated the normal skin area 0.5 or 1 cm from the margin of the operative suture lines. The surrounding normal tissue was shielded with lead (equivalent to 6 mm of Pb). The total treatment doses administered ranged from 12 to 18 Gy: 12 Gy (biological effective dose [BED] 15.6 Gy) for 11 lesions, 15 Gy (BED 19.5 Gy) for 20 lesions, 16 Gy (BED 22.4 Gy) for 3 lesions, and 18 Gy (BED 23.4 Gy) for 3 lesions. The treatment dose distribution by site was as follows: 12 Gy for 9 lesions in the ear lobe and 2 lesions in the helix of the auricle; 15 Gy for 13 lesions in the ear lobe, 4 lesions in the helix of the auricle, 2 lesions in the chest wall, and 1 lesion in the shoulder; 16 Gy for 1 lesion in the chest wall, 1 lesion in the shoulder, and 1 lesion in the abdomen; 18 Gy for 2 lesions in the shoulder and 1 lesion in the abdomen. Radiation therapy was administered every other day at 3 or 4 Gy. Radiation therapy was initiated within 24 h for 24 lesions, between 24 and 72 h for 6 lesions, and more than 72 h after surgical excision for 7 lesions (Table 2).
In this study, the recurrence-free success rate was 81.1% during the follow-up period (Fig. 1). In our cohort of 30 keloid patients with 37 lesions who received postoperative radiation therapy, 7 lesions recurred (Fig. 2). The median to recurrence was 7.9 months (6~13 months). Of the 7 lesions, 4 recurred in male patients and 3 recurred in female patients. Areas of recurrence were the ear lobe (1 lesion), the helix of the auricle (1 lesion), the shoulder (2 lesions), the chest wall (2 lesions), and the abdomen (1 lesion). The radiation doses administered to the 7 recurred lesions were 12 Gy (2 lesions), 15 Gy (2 lesions), 16 Gy (1 lesion), and 18 Gy (2 lesions). The timing of radiation treatment after surgical keloid removal was within 24 h for 1 lesion, and after more than 72 h for 6 lesions (p<0.0001).
Five of the 7 recurred lesions appeared in high stretch-tension regions including the shoulder, chest wall, and abdomen while the remaining 2 lesions appeared in low stretch-tension regions including the ear lobe and helix of the auricle (p=0.010) (Table 3).
Sex, causative factors, age, family history, surgical methods, and total treatment dose were not associated with keloid recurrence to a statistically significant degree.
Of the 30 patients (37 lesions), 19 (22 lesions) had a family history (p=0.250). Among these maternal and paternal hereditary tendencies were observed in 14 (16 lesions) and 5 (6 lesions) patients, respectively (p=0.033). In the overall 30 patient cohort, family history was positive in 5 of the 7 patients who developed a recurrence and 14 of the 23 patients who did not (p=0.677) (Table 4). Sex, causative factors, lesion location, and age were not statistically associated with keloid development.
During treatment, mild skin erythema (grade 1 dermatitis7) was observed in 8 lesions (1 lesion in the ear, 1 lesion in the helix of the auricle lesion, 2 lesions in the chest wall, 2 lesions in the shoulder, 2 lesions in the abdomen), and these symptoms disappeared within a few weeks of treatment. Surgical wounds dehiscence did not occur. During the follow-up period, transient hyperpigmentation was observed in 3 treated lesions (1 lesion in the shoulder, 2 lesions in the abdomen) and the symptoms disappeared within 4 months of treatment. Secondary carcinogenesis did not occur during the follow-up period.
Keloids result from defective negative feedback mechanisms in fibroblasts during the wound healing process. They occur outside excessive collagen depositions and are diseases owing to the decomposition of matrix metalloproteinases1,9,10. Keloid occur in contrast to the normal wound healing process, which regulates cell growth and proliferation via changes in cellular signals: keloids have also been known to occur during the wound healing process1,3,11. Compared to normal fibroblasts, keloid-derived fibroblasts are immature and exhibit increased production of type I or III collagen and matrix metalloproteinases. The result is an overgrowth of type III collagen at the wound site, which is then slowly replaced by type I collagen, unlike the normal wound healing process1,3,11. The abnormal expression of various growth factors and related receptors has been associated with keloid-derived fibroblasts12,13. In recent studies, keloid-derived fibroblasts have been shown to overexpress the following growth factors: vascular endothelial growth factor, transforming growth factor (TGF)-beta 1, TGF-beta 2, connective tissue growth factor, the platelet-derived growth factor receptor. In particular, TGF-beta 1, a cytokine that participates in wound healing and fibrosis, is an important component in the formation of keloids and in histamine, which is considered capable of accelerating collagen formation during the proliferation phase of wound healing1,3,4,12,13.
Postoperative radiotherapy is expected to favorably impact wound healing in patients with keloids at a time when the connective tissue is more radiosensitive by decreasing fibroblast proliferation and causing a rapid degranulation of mast cells the main secretors of histamine1,14,15. Radiation suppresses collagen synthesis in keloids or hypertrophic scars. This is mediated by the inhibition of TGF-beta 1 release from fibroblasts. Otherwise, histamine released from mast cells stimulates the proliferation of fibroblasts. Radiation inhibits histamine release from mast cells, which in turn inhibits the proliferation of fibroblasts. Thus, the mechanism by which radiation inhibits keloids or hypertrophic scars is the suppression of fibroblast proliferation and inhibition of collagen synthesis14,16.
Considering that the proliferation phase occurs 2 or 3 days (48~72 h) after an injury, radiation therapy should be initiated within 72 h after keloid surgery. In this study, initial radiation treatment occurred more than 72 h after surgery in 6 of the 7 recurred lesions (p<0.0001).
Several authors14,15 have reported the excessive proliferation of collagen fibers in keloid scars with high stretch-tension. Repeated hyperextension stimulation has been performed on collagen overgrowth at treated keloid sites14,15. Bischof et al.15 reported recurrences at sites of high stretch-tension, and these were relatively common. In this study, 5 of the 7 recurred lesions appeared in high stretch-tension regions (the shoulder, chest wall and abdomen) and the remaining 2 lesions appeared in low stretch-tension regions (the ear lobe and helix of the auricle). This difference was statistically significant (p=0.010). Keloid occurrence and recurrence as well as the presence of a family history have been examined in numerous studies within contradictory results. Some authors have reported that both the occurrence and recurrence of keloids is associated with family history, while others reported that family history is not significantly related to keloids. In our study, 19 of the 30 keloid patients who received postoperative radiation therapy had family histories and 11 patients did not (p=0.250). Family history was present in 5 of the 7 patients who displayed recurrence and in 14 of the 23 patients who did not. This difference was not statistically significant (p=0.677). However, in a detailed analysis of genetic predisposition among those with family histories, a maternal inherited genetic predisposition was significantly related to the occurrence of keloids (p=0.033). During fetal development, actin, fibronectin, and ribosomes are known to be inherited mainly from the mother17, and these genetic factors have been associated with keloid development in laboratory micro-array findings from several studies17,18. Satish et al.19 reported micro-array data comparing the gene expression profiles of keloid and normal tissues. They found increased expression levels of fibronectin, several actin isoforms, and the alpha-1 chain of type I collagen proteins, which were all associated with abnormal wound healing20. In addition, some tumor-related genes were found to be up-regulated in keloid-derived fibroblasts, with greater increases observed for ribosomal protein 18, an important protein for cell growth, and Stat-3, another oncogene involved in cell proliferation19,20,21. These in vitro findings, concerning the extracellular matrix and several genes that interact with the complex process of wound healing in general cannot explain the exact genetic relationship but factors related to the link with maternal genetics were observed.
The recurrence rate in our study was 18.9%. The acute side effect of radiation dermatitis was observed in 21.6% of the cases, and chronic side effects were observed in 8.2% of the cases. However, all side effects disappeared during the follow up period. Recurrence rates between 15% and 32.7% have been reported in other studies7,18. Kovalic and Perez22 monitored 75 patients with 113 treated scars and found an overall local control rate of 73%. Meanwhile, Borok et al.23 reported excellent cosmetic results without recurrence in 92% of cases. The results of our study can be considered satisfactory in comparison to those of other studies.
Postoperative electron beam radiotherapy can be regarded as an effective treatment method for the prevention of keloid recurrence without serious adverse events. However, radiation therapy should be initiated within 72 h after surgical excision to prevent recurrence. Recurred lesions were more common in high stretch-tension regions than in low stretch-tensions, and this difference was statistically significant. The occurrence of keloids was associated with family history, but this was not statistically significant. However, a significant tendency towards maternal genetic predisposition was observed. Combination therapy with anti-collagen synthesis drugs or silicone sheets should be considered for high stretch-tension areas to reduce stretch tension and inhibit collagen synthesis. Moreover, careful monitoring is necessary in patients with maternal genetic predispositions for keloids.
Figures and Tables
Fig. 1
(A) A lesion limited to the right posterior ear lobe was observed prior to surgery. (B) A recurrent lesion was not observed during the follow-up period after radiotherapy.

Fig. 2
(A) A lesion was observed on the left ear lobe. (B) Radiotherapy was performed 7 days after surgery. A small, recurrent, hypervascular lesion re-grew after radiotherapy. Steroid injection therapy was administered to the recurrent lesion.
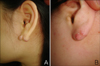
Table 1
Characteristics of 30 keloid patients with 37 lesions who received postoperative radiation therapy

Table 2
Radiation dose and initial time of treatment in 30 keloids patients with 37 lesions who received postoperative radiation therapy

References
1. Marneros AG, Krieg T. Keloids--clinical diagnosis, pathogenesis, and treatment options. J Dtsch Dermatol Ges. 2004; 2:905–913.
2. Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001; 17:263–272.

3. Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004; 26:379–384.

4. Jung JY, Roh MR, Kwon YS, Chung KY. Surgery and perioperative intralesional corticosteroid injection for treating earlobe keloids: a korean experience. Ann Dermatol. 2009; 21:221–225.

5. Clark JA, Turner ML, Howard L, Stanescu H, Kleta R, Kopp JB. Description of familial keloids in five pedigrees: evidence for autosomal dominant inheritance and phenotypic heterogeneity. BMC Dermatol. 2009; 9:8.

6. Omo-Dare P. Genetic studies on keloid. J Natl Med Assoc. 1975; 67:428–432.
7. Ogawa R, Mitsuhashi K, Hyakusoku H, Miyashita T. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003; 111:547–553. discussion 554-555.

8. van de Kar AL, Kreulen M, van Zuijlen PP, Oldenburger F. The results of surgical excision and adjuvant irradiation for therapy-resistant keloids: a prospective clinical outcome study. Plast Reconstr Surg. 2007; 119:2248–2254.

9. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004; 36:1031–1037.

10. Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004; 2:E7.

11. Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005; 304:274–286.

12. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005; 153:295–300.

13. Topol BM, Lewis VL Jr, Benveniste K. The use of antihistamine to retard the growth of fibroblasts derived from human skin, scar, and keloid. Plast Reconstr Surg. 1981; 68:227–232.

14. Caccialanza M, Piccinno R, Schiera A. Postoperative radiotherapy of keloids: a twenty-year experience. Eur J Dermatol. 2002; 12:58–62.
15. Bischof M, Krempien R, Debus J, Treiber M. Postoperative electron beam radiotherapy for keloids: objective findings and patient satisfaction in self-assessment. Int J Dermatol. 2007; 46:971–975.

16. Rosen EM, Goldberg ID, Myrick KV, Levenson S. Radiation survival properties of cultured vascular smooth muscle cells. Radiat Res. 1984; 100:182–191.

17. Kingston HM. Genetics assessment and pedigree analysis. In : Rimoin DL, Connor JM, editors. Emery and Rimoin's principles and practice of medical genetics. 5th ed. Philadelphia: Churchill Livingstone;2007. p. 518–535.
18. Marneros AG, Norris JE, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol. 2001; 137:1429–1434.

19. Satish L, Lyons-Weiler J, Hebda PA, Wells A. Gene expression patterns in isolated keloid fibroblasts. Wound Repair Regen. 2006; 14:463–470.

20. Lim CP, Phan TT, Lim IJ, Cao X. Stat3 contributes to keloid pathogenesis via promoting collagen production, cell proliferation and migration. Oncogene. 2006; 25:5416–5425.

21. Bayat A, Bock O, Mrowietz U, Ollier WE, Ferguson MW. Genetic susceptibility to keloiddisease and transforming growth factor beta 2 polymorphisms. Br J Plast Surg. 2002; 55:283–286.

22. Kovalic JJ, Perez CA. Radiation therapy following keloidectomy: a 20-year experience. Int J Radiat Oncol Biol Phys. 1989; 17:77–80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download