Abstract
Background
Measuring the quality of life (QOL) is important in the evaluation of nonclinical aspects of diseases, for the discovery of functional and psychological limitations, and in choosing treatment in the initial phase of the disease. Pemphigus is a potentially fatal autoimmune bullous disease caused by autoantibodies against desmogleins (cadherin family proteins in desmosomes). Thus far, there has been no published study on QOL in Korean patients with pemphigus.
Methods
Sixty-six patients enrolled at the Gangnam Severance Hospital from March 2012 to March 2013 were assessed for QOL by using the Dermatology Life Quality Index (DLQI), and for anxiety and depression by using the General Health Questionnaire (GHQ). Spearman's rank-order correlation, t-test, and ANOVA were used to identify the relations between the DLQI score and other clinical variables.
Results
Pemphigus vulgaris and pemphigus foliaceus significantly reduced the QOL of patients. The average DLQI score for all patients was 10.18. The mean DLQI score was 13.45 in patients in the active state and 5.15 in the patients in the remission state. The DLQI score highly correlated with disease severity, titer of anti-desmoglein 1 in enzyme-linked immunosorbent assay, and the corticosteroid dose. However, the QOL was not affected by sex, age, subtype of pemphigus, duration of disease, or comorbidities. Forty-two percent of the patients showed a positive result in the GHQ, reflecting probable minor psychiatric nonpsychotic conditions, and the GHQ score positively correlated to the DLQI score.
There are many definitions for the term "quality of life" (QOL). The World Health Organization has defined QOL as individuals' perceptions of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards, and concerns1. The QOL includes the physical, functional, social, and emotional well-being of a person. In addition, measuring the QOL is important when evaluating the benefits and harms of a treatment. It is also used for the improvement of the doctor-patient relationship. Therefore, in recent years, dermatologists have leaned toward using QOL measures as an adjunct to the clinical assessment of diseases, as a secondary outcome in clinical practice2. Although skin diseases are rarely life-threatening, their impact on the QOL can be major. For instance, the impact of psoriasis on the QOL has been found to be comparable to the impact of heart failure3.
Pemphigus is a rare but severe autoimmune bullous disease caused by autoantibodies directed against desmogleins, and it is clinically characterized by bullae and painful erosions of the skin and mucous membranes4. The different types of pemphigus can be distinguished by the specificity of autoantibodies to different targets or by the location of blister formation. Generally, pemphigus can be divided into two major forms: pemphigus vulgaris (PV) and pemphigus foliaceus (PF). Patients with PV, which is associated with autoantibodies against desmoglein 3 and 1, have mucous membrane erosions and/or cutaneous blisters. PF patients exclusively have cutaneous blisters without mucosal lesion and are associated with autoantibodies against desmoglein 1. Other subtypes of the pemphigus group include paraneoplastic pemphigus, IgA pemphigus, and drug-induced pemphigus.
Until now, there have been only a few published studies on the QOL in patients with pemphigus56789101112. All of these studies have demonstrated a great impact on the QOL and psychological status of pemphigus patients. In particular, patients with severe skin and mucosal involvement experience a more impaired QOL than others. The aim of this study is to determine the QOL in a large number of Korean patients with pemphigus. In this study, we used the Dermatology Life Quality Index (DLQI) and the General Health Questionnaire (GHQ) to evaluate the QOL and psychological status of each patient.
A single-center, cross-sectional study was performed from March 2012 to March 2013 at the Department of Dermatology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Informed consent was obtained from all patients after providing them with written and oral information. The study was approved by the institutional review board of the Gangnam Severance Hospital (IRB No. 3-2012-0012).
The criteria for patient selection were as follows: patients with PV or PF whose diagnoses were confirmed by clinical features, pathology, and direct immunofluorescence findings; patients who were able to provide written informed consent; patients aged 16 years or older; patients able to read the Korean language; and patients who did not have any severe dermatological, mental, or physical illness.
The information collected included personal data (e.g., age, sex, body mass index, marital status, educational level, employment status, number of children, and history of cigarette smoking or alcohol use), clinical data (e.g., comorbidities, date of onset, clinical subtype, and clinical activity of disease), Pemphigus Disease Area Index (PDAI) score, immunological data concerning autoantibodies, and prescribed treatment.
Concerning disease activity, we used the consensus statement on the definition of disease activity and therapeutic response proposed by the International Pemphigus Committee in 200813. The definitions of complete remission and partial remission are the absence of new or established lesions for at least 2 months and the presence of transient new lesions that heal within 1 week, respectively.
Patients were examined by two dermatologists to assess the disease severity by using the PDAI scoring system, which was developed by the International Pemphigus Definition Committee13. This scoring system has three components relating to the skin, scalp, and mucous membranes. In the skin assessment, 12 anatomic sites (ears, nose, face, neck, chest, abdomen, back and buttocks, arms, hands, legs, feet, and genitals) are reviewed and assigned a score according to the following disease extent: 0 (no lesions), 1 (1~3 lesions, all ≤6 cm; up to 1 lesion >2 cm), 2 (2~3 lesions, all ≤6 cm; at least 2 lesions >2 cm), 3 (>3 lesions, all ≤6 cm), 5 (>3 lesions and/or 1 lesion >6 cm), or 10 (>3 lesions and/or at least 1 lesion >16 cm). The scalp is assigned a score based on the presence of bullae, erosions, or new erythema of 0 (no activity), 1 (one quadrant affected), 2 (two quadrants affected), 3 (three quadrants affected), 4 (whole scalp affected), or 10 (at least 1 lesion >6 cm). Mucosal activity is assessed by reviewing 12 mucosal sites (eyes, nose, buccal, hard palate, soft palate, upper gingiva, lower gingiva, tongue, floor of the mouth, labial mucosa, posterior pharynx, and anogenitalia) and assigning a score based on the presence of erosions and blisters as follows: 0 (absent), 1 (1 lesion), 2 (2~3 lesions), 5 (>3 lesions or 2 lesions >2 cm), or 10 (entire area). The total possible score for the PDAI ranges from 0 to 130 for the skin score (120 points for body, 10 points for scalp) and up to 120 points for mucosal activity.
The DLQI is one of the most frequently used QOL measures in dermatology. It consists of 10 questions and is designed for use in adults older than 16 years. The questions are classified in the following subscales: symptoms and feelings (questions 1 and 2), daily activities (questions 3 and 4), leisure (questions 5 and 6), personal relationships (questions 8 and 9), work and school (question 7), and treatment (question 10). The DLQI score is calculated by summing the scores of the 10 questions. The maximum score is 30, and the minimum score is 0. The higher the score, the more the QOL is impaired. Grade 1 (0~1) means no effect at all on the patient's life. Similarly, grade 2 (2~5) means small, grade 3 (6~10) means moderate, grade 4 (11~20) means very large, and grade 5 (21~30) means an extremely large effect on the patient's life13. In this study, we implemented a validated Korean translation of the DLQI.
The GHQ-12 is a self-administered 12-item questionnaire designed to measure psychological distress and to detect current nonpsychotic psychiatric disorders such as depression and anxiety. Answers are given on a four-point scale. When scored with the binary method (0-0-1-1), the GHQ-12 can be used as a screening tool to detect minor nonpsychotic psychiatric disorders, yielding final scores that range from 0 to 12. Operationally, patients scoring ≥4 are considered "GHQ-positive," reflecting probable minor nonpsychotic psychiatric disorders like depression and anxiety.
Statistical analyses were performed by using commercial software (SAS ver. 9.2; SAS Institute, Cary, NC, USA). Descriptive data are presented as numbers with percentages and means with standard deviations. Spearman's rank-order correlation, t-test, ANOVA, and multiple regression analysis were used to identify the relations between the DLQI score and other clinical variables. Differences were defined as statistically significant at p<0.05.
A total of 66 patients (31 male, 35 female) with an average age of 51.1±13.5 years were included. Forty patients had PV and 26 patients had PF. The average duration of disease was 45.7±38.1 months. Forty patients were in the active state, and 26 patients were in the remission state.
The average DLQI score for all patients was 10.2±8.8. The most impaired element of the QOL was clinical symptoms and feelings, whereas treatment was the least affected. Table 1 shows more details on the different variables and DLQI scores.
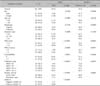
We observed a positive correlation between the PDAI and DLQI scores (r=0.71, p<0.0001). In addition, the mucosal PDAI score was correlated with the DLQI score (r=0.36, p=0.003). The dose of the systemic corticosteroid (methylprednisolone [MPD]) showed a strong positive correlation with the DLQI score (r=0.70, p<0.0001). The titer of anti-desmoglein 1 antibody correlated with DLQI score (r=0.60, p=0.001), but that of anti-desmoglein 3 antibody did not. Other variables showed no statistically significant correlation with the DLQI score (Table 2).
No significant differences in the DLQI score were observed between PV and PF, sex, or duration of disease (Table 3). The DLQI scores of PV and PF patients were 9.93 and 10.58, respectively. Furthermore, there were no significant differences in the scores between PV and PF in the active state or the remission state. The DLQI scores of PV and PF patients in the active state were 12.96 and 14.27, respectively, and those in the remission state were 4.87 and 5.55, respectively. Patients who were treated with a higher dose of corticosteroid (>10 mg MPD) showed a statistically significant increase in the DLQI score compared with those with a lower corticosteroid dose. The average PDAI score of the lower-dose MPD group was 5.03 and that of the higher-dose MPD group was 31.96 (p< 0.0001). The use of an adjuvant immunosuppressive agent did not significantly affect the QOL of the patients (Table 3). The clinical activity of the disease also affected the DLQI of pemphigus patients (Table 3). Patients who achieved partial remission and complete remission showed statistically significantly decreased DLQI scores compared with patients with active disease (p<0.0001); however, no statistically significant difference was observed between patients with partial remission and those with complete remission (p=0.2751). The mean DLQI score of the active group was 13.45±9.3, whereas that of the complete and partial remission groups was 5.15±4.8 (Table 3). We estimated the titer of autoantibodies with enzymelinked immunosorbent assay. The titer of anti-desmoglein 1 antibody, which was estimated in 27 patients, correlated with the DLQI score (r=0.60, p<0.001); however, the titer of anti-desmoglein 3 antibody, which was estimated in 25 patients, did not correlate with the DLQI score (r=0.23, p<0.279).
A positive GHQ, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was detected in 47% of the patients. GHQ positivity was associated with a higher DLQI score (p<0.0001). A significant GHQ positivity difference was observed between patients in the active state and those in the remission state (p=0.0085).
Pemphigus is a rare, severe, and potentially fatal disease with a strong negative effect on both dermatological and general health. Despite this serious condition, only a few studies on QOL in pemphigus patients have been published. Moreover, no QOL study on pemphigus patients in Korea has been reported. Therefore, this study is meaningful in that we measured the QOL of Korean pemphigus patients and compared the results with the QOL scores reported in other studies.
We decided to use a skin-specific QOL measurement tool, the DLQI, to facilitate comparison with other dermatologic conditions. The DLQI was derived from patients with various skin diseases and provides a simple method of scoring the impact of skin disease on the QOL. A higher score indicates a greater impairment of the QOL. Healthy persons have an average score of 0.514. Two studies used the DLQI for evaluating the QOL of pemphigus patients611. Both of these studies showed a significant decrease in the QOL; the DLQI score of each study was 10±6.6 and 10.98±5.9, respectively. In our study, the average DLQI score of pemphigus patients was 10.18±8.8, which is similar to that of the previous two studies. One study estimated the DLQI score of epidermolysis bullosa (EB), which is another life-threatening bullous disease15. The mean DLQI score of adult EB simplex patients was 10.7, which was similar to that of our pemphigus patients; however, the score of adult patients with the Hallopeau-Siemens subtype of dystrophic EB (18.0) was extremely higher than that of pemphigus patients. Atopic dermatitis and psoriasis are representative chronic dermatologic disorders. Although the QOL scores of these skin diseases have been reported variably in each paper, in a recent large cohort study, the measured DLQI scores of atopic dermatitis and psoriasis were 7.31±5.98 and 5.93±5.66, respectively16. Many negative factors, including the mucosal involvement, painful erosive cutaneous lesions, more aggressive systemic corticosteroid treatment and its adverse effects, and higher mortality might be the reasons for the more impaired QOL in pemphigus patients.
Terrab et al.7 used the Medical Outcome Study 36-item Short-Form Survey (SF-36) questionnaire, which is a generic QOL measurement tool, to compare the QOL in 30 pemphigus patients and 60 healthy adults. Pemphigus patients showed an impaired QOL compared with healthy controls, particularly in those with facial involvement and a large extent of lesions. Tabolli et al.8 measured the QOL of 58 pemphigus patients with the SF-36 and observed significantly worse QOL in patients with mucocutaneous involvement, severe pemphigus, and pemphigus of recent onset. Paradisi et al.9, who used the SF-36 and Skindex-29 questionnaires, reported that a more impaired QOL was observed in patients who had both mucous and cutaneous involvements. In addition, comorbidities and female sex were associated with worse QOL.
In this study, we used the PDAI scoring system to evaluate the severity of disease. The PDAI scoring system, which was developed by the International Pemphigus Definition Committee, has not been used in previous pemphigus QOL studies13. The PDAI scoring system is recognized to be more reproducible than the Autoimmune Bullous Skin Disorder Intensity Score, which is also a widely used severity score index, and is able to detect smaller differences17. Other crude score indices, such as the Ikeda index and Physician's Global Assessment, are not as reliable or as accurate as the PDAI17. Like other previous pemphigus QOL studies789, our study showed that the DLQI score highly correlated with the clinical severity of the disease. In addition, the mucosal PDAI score, which shows the severity of mucosal involvement, also correlated with the DLQI score. Mucosal involvement of the disease is most burdensome in pemphigus patients because mucosal lesions have a larger impact on the patients' fundamental and essential activities such as speaking and eating.
Clinical activity as defined by the International Pemphigus Committee in 200813 also affects the QOL of pemphigus patients. According to the definition, clinical activity is intimately associated with disease severity. As mentioned above, clinical severity measured by the PDAI scoring system significantly correlated with the QOL of patients. Therefore, patients with remission who had few or no skin lesions showed more improved QOL than the group who had active skin lesions; however, there was no statistical difference in the DLQI scores between patients with partial remission and those with complete remission.
Treatment was one of the major factors that associated with the QOL of patients. Patients who were treated with low-dose MPD (≤10 mg) showed a higher QOL. This may be because severe skin or mucosal involvement requires a high dose of corticosteroid, which can cause various adverse effects. We divided the pemphigus patients into the lower-dose corticosteroid (≤10 mg) group and the higher-dose corticosteroid (>10 mg) group. The average PDAI score of the lower-dose corticosteroid group was 5.03 and that of the higher-dose corticosteroid group was 31.96 (p<0.0001). These results indicate that the dose of corticosteroid correlated to clinical severity. Therefore, it is more likely that patients with low-dose corticosteroid have a higher QOL because of less disease activity. All of the patients with adjuvant therapy were treated with mycophenolate mofetil (MMF), except for two patients, because MMF is known to have a better safety profile than other immunosuppressive agents18. The use of an adjuvant immunosuppressive agent did not affect the QOL of the patients in our study. A recently published study also showed no significant difference in QOL impairment between patients who were treated with an adjuvant immunosuppressive agent and those who were not10. Compared with systemic corticosteroids, the dose of MMF showed less correlation with disease severity. Therefore, the use of MMF in patients with pemphigus might not significantly affect the QOL of the patients.
We expected that the titer of autoantibody will correlate with the DLQI score, especially anti-desmoglein 3 antibody, which is a main cause of mucosal involvement. Both the titers of anti-desmoglein 1 and anti-desmoglein 3 antibodies showed a positive correlation with the DLQI; however, there was no statistically significant correlation between the titer of anti-desmoglein 3 antibody and the DLQI score. The reason for this unexpected result is unclear; however, the titer of autoantibody might not finely reflect the clinical status.
In this study, the QOL was not affected by sex, age, subtype of pemphigus, or duration of disease. Higher DLQI scores were observed in patients with a more recent onset compared with patients with a longer disease duration, although this difference did not reach statistical significance. Tabolli et al.8 pointed out that a recent diagnosis and limited access to information about the clinical implications of pemphigus may dramatically influence the patients' QOL. On the other hand, over time, decreased symptoms and a decreased dose of corticosteroid may increase the QOL of pemphigus patients.
Previous studies revealed that pemphigus is highly associated with psychological problems8911. Although the DLQI has been widely used to evaluate the QOL in various cutaneous diseases, this index does not fully encompass the emotional and mental health aspects that might be affected. Therefore, the GHQ-12 was used to evaluate the nonpsychotic psychiatric problems of pemphigus patients in this study. The prevalence of patients who were GHQ positive was 47%, which was very high considering that the prevalence in the general population is 10% to 12%1. The positive rate of GHQ-12 in our patients was similar to that of a previous study performed by Paradisi et al.9 There are some studies demonstrating that psychological factors are related to the development of autoimmune disease1920. Another interesting finding of this study was a positive correlation between the DLQI and GHQ-12 scores. We adjusted the PDAI score to exclude any influence of clinical severity; however, the DLQI score still correlated with the GHQ-12 score (p<0.0001). As this study is not a controlled trial, it is difficult to determine a cause-effect relation between clinical severity and psychological distress. Ghodsi et al.11 reported similar results to our study and insisted that the QOL was influenced by psychiatric comorbidities. It is unclear whether psychological distress contributed to the onset and progression of pemphigus; however, the psychological states of pemphigus patients should be considered during treatment.
This is a cross-sectional study in which patients were observed at a single time point during the course of the disease. This limitation makes it difficult to establish a clear cause-effect relation between multiple variables. Additional long-term follow-up observation should be provided to monitor the variation in QOL during treatment and to determine other variables that may affect the QOL of pemphigus patients.
In conclusion, pemphigus had a significant impact on QOL and psychological status. The most important factors that affect QOL were found to be the clinical severity of pemphigus as measured by the PDAI and the therapeutic dose of corticosteroid. Therefore, consideration should be given to patients' QOL and psychological states, as well as clinical states, for an effective care and patient satisfaction.
Figures and Tables
Table 2
Correlation between various clinical characteristics and Dermatology Life Quality Index (DLQI) score and General Health Questionnaire (GHQ)-12 by using Spearman's rank correlation

References
1. Regier DA, Boyd JH, Burke JD Jr, Rae DS, Myers JK, Kramer M, et al. One-month prevalence of mental disorders in the United States. Based on five Epidemiologic Catchment Area sites. Arch Gen Psychiatry. 1988; 45:977–986.

2. Anderson RT, Rajagopalan R. Development and validation of a quality of life instrument for cutaneous diseases. J Am Acad Dermatol. 1997; 37:41–50.

3. Abeni D, Picardi A, Pasquini P, Melchi CF, Chren MM. Further evidence of the validity and reliability of the Skindex-29: an Italian study on 2,242 dermatological outpatients. Dermatology. 2002; 204:43–49.

4. Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005; 14:861–875.

5. Masahiro S, Shigaku I, Yutaka I, Ogawa H. An investigation of quality of life (QOL) of pemphigus patients in Japan (First report). Jpn J Dermatol. 2000; 110:283–288.
6. Mayrshofer F, Hertl M, Sinkgraven R, Sticherling M, Pfeiffer C, Zillikens D, et al. Significant decrease in quality of life in patients with pemphigus vulgaris. Results from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005; 3:431–435.

7. Terrab Z, Benchikhi H, Maaroufi A, Hassoune S, Amine M, Lakhdar H. Quality of life and pemphigus. Ann Dermatol Venereol. 2005; 132:321–328.
8. Tabolli S, Mozzetta A, Antinone V, Alfani S, Cianchini G, Abeni D. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008; 158:1029–1034.

9. Paradisi A, Sampogna F, Di Pietro C, Cianchini G, Didona B, Ferri R, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009; 60:261–269.

10. Paradisi A, Cianchini G, Lupi F, Di Pietro C, Sampogna F, Didona B, et al. Quality of life in patients with pemphigus receiving adjuvant therapy. Clin Exp Dermatol. 2012; 37:626–630.

11. Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, Valikhani M, Esmaili N. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012; 39:141–144.

12. Tabolli S, Pagliarello C, Paradisi A, Cianchini G, Giannantoni P, Abeni D. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014; 170:1087–1091.

13. Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008; 58:1043–1046.

14. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994; 19:210–216.

15. Horn HM, Tidman MJ. Quality of life in epidermolysis bullosa. Clin Exp Dermatol. 2002; 27:707–710.

16. Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000; 80:430–434.

17. Daniel BS, Hertl M, Werth VP, Eming R, Murrell DF. Severity score indexes for blistering diseases. Clin Dermatol. 2012; 30:108–113.

18. Meurer M. Immunosuppressive therapy for autoimmune bullous diseases. Clin Dermatol. 2012; 30:78–83.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download