Abstract
Background
The treatment options for oral lichen planus (OLP) are numerous and include topical and systemic agents. Intralesional and systemic corticosteroids are used; however, the therapeutic results are often disappointing.
Objective
To compare the influence of ozone, laser, and topical corticosteroid therapies in the treatment of OLP.
Methods
One hundred twenty adult patients with ≤3 cm atrophic-erosive biopsy-proven OLPs in the tongue or buccal mucosa were recruited into the study. They were randomly assigned, by preoperative envelope drawing, to be treated with low-level laser therapy (LLLT group), ozone therapy (ozonated group), and topical corticosteroid therapy (positive control group). A placebo treatment containing base ointment without the active corticosteroid component was administered to patients in the negative control group. Response rate scores were determined on the basis of changes in the appearance score and pain score of the lesions between baseline and after each treatment.
Results
The study subjects consisted of 56 male and 64 female OLP patients with a combined mean age of 42.6±8.3 years (range, 28~55 years). No statistically significant difference was detected in clinical severity among the groups. The sign scores decreased in almost all scoring groups; however, statistically significant improvement was found in the ozonated and corticosteroid-treated groups. Symptom improvement was achieved after treatment with LLLT, ozone, and corticosteroid (p<0.05). The efficacy indices were significantly higher in the ozonated and corticosteroid-treated groups.
Lichen planus is a common chronic mucocutaneous disease of uncertain origin that has been shown to affect 0.5% to 2.2% of the studied populations12345678. The oral lichen planus (OLP) affects approximately 2% of the population23. OLP, in general, may arise in >70% of persons with skin lesions. The frequency of malignant change ranges from 0.4% to 3.3%, with the periods of observation being from 0.5 to >20 years4.
Six clinical forms of OLP have been described: reticulated, plaque-like, erosive, papular, atrophic, and bullous4. Reticular OLP is the most common form and is relatively asymptomatic. On the other hand, the erosive, atrophic, and bullous forms are typically the most symptomatic, often debilitating, and prompt the patient to seek care. Compared with self-limiting cutaneous lesions, most OLP lesions are chronic, rarely undergo spontaneous remission, and are difficult to treat completely56.
OLP is seen worldwide, mostly in the fifth to sixth decades of life, and is twice as prevalent in women as in men14. The differential diagnosis of erosive OLP includes squamous cell carcinoma, chronic candidiasis, benign mucous membrane pemphigoid, pemphigus vulgaris, chronic cheek chewing, lichenoid reaction to dental amalgam or drugs, hypersensitivity mucositis, and systemic disease such as erythema multiforme, graft-versus-host disease, and discoid lupus erythematosus347.
The treatment options for OLP are numerous and include topical and systemic agents. Intralesional and systemic corticosteroids are used but with often disappointing therapeutic results48. Topical corticosteroids remain the mainstay of therapy; however, their long-term use may cause some adverse effects such as candida overgrowth, thinning of the oral mucosa, and discomfort on application. Gorsky et al.9 showed that candidal lesions were found in 32% of OLP patients who received corticosteroid therapy. In addition, some patients may not respond effectively to only topical corticosteroid application.
Low-level laser therapy (LLLT) has potential biostimulating effects when applied to oral mucosal tissues. LLLT seems to offer some benefits in controlling the inflammatory process by promoting the healing of the tissues, but without undesired adverse effects, and also by reducing pharmacologic support after a surgery10.
The anti-inflammatory effect of LLLT could be due to an increase of phagocytic activity, an increase in the number and diameter of lymphatic vessels, a decrease in the permeability of blood vessels, and a restoration of microcapillary circulation, normalizing the permeability of vascular walls and decreasing edema1112. LLLT has also gained acceptance for the treatment of premalignant oral mucosal lesions such as leukoplakia and OLP. Many types of laser are now used for the treatment of various diseases. A few literature reports demonstrated the use of a diode laser for the treatment of OLP1314.
Another nonmedication method used to treat OLP in dentistry is ozone therapy15. Ozone reacts with blood components (erythrocytes, platelets, leukocytes, and endothelial cells) and induces oxygen metabolism, cell energy, immunomodulatory changes, the antioxidant defense system, and microcirculation in tissues16. Such effects resemble the biostimulatory property of LLLT that has been widely studied1017.
The aim of this study is to compare the influence of ozone, laser, and topical corticosteroid therapies in the treatment of atrophic-erosive OLP.
One hundred twenty adult patients with atrophic-erosive OLP (≤3 cm) in the tongue or buccal mucosa were recruited into the study. The OLP was diagnosed clinically and histopathologically. The patients were randomly assigned, by preoperative envelope drawing, to be treated with LLLT (laser group), ozone therapy (ozonated group), or topical corticosteroid (positive control group). A placebo treatment containing base ointment without the active corticosteroid component was administered to patients in the negative control group. Each group consisted of 30 patients.
The exclusion criteria were as follows: 1. Presence of systemic diseases that may cause OLP, such as hepatitis C; 2. age <20 years; 3. pregnant or breastfeeding; 4. use of lichenoid reaction-inducing drugs such as antihypertensives, diuretics, nonsteroidal anti-inflammatory drugs, anticonvulsants, and drugs for treating tuberculosis; 5. presence of histologic signs of dysplasia in the biopsy specimen; 6. previous OLP treatment within 1 month before the beginning of the study; 7. lesions adjacent to the amalgam filling site; and 8. systemic corticosteroid use.
All patients were informed about the potential complications of laser, ozone, and corticosteroid treatments and each gave written consent on an institutionally approved form. This study followed the medical protocol and ethics of the Declaration of Helsinki. Ethical approval was obtained from Bezmialem Vakif University's ethical committee (IRB No. 34/16).
Patients in the LLLT group were treated with laser irradiation (exposure time, 2.5 min; fluence, 1.5 J/cm2 per session; irradiance, 10 mW/cm2; no. of illumination point, 1; area, 1 cm2). A diode laser (808 nm, 0.1 W, continuous wave; Fotona XD-2; Fotona, Ljubljana, Slovenia) was used as a light source. A light exposure dose of 120 J/cm2 was used for 2.5 min. The lesions and 0.5 cm of their surrounding tissue were illuminated with a spot size of 1 cm2. Laser irradiation was done two times a week (once every third day) for a maximum of 10 sessions. In each session, the laser used was not in contact with the tissues. The application distance was 0.5~1 cm; because at this distance, the difference in application distance did not affect the spot size with the handpiece that was used. Large lesions were illuminated with multiple spots.
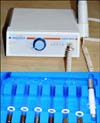
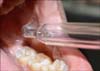
Ozone therapy was performed by using an ozone generator (Ozonytron; Biozonix GmbH, Munich, Germany) with a tissue probe (alveolar probe) (Fig. 1). The ozone generator was applied intraorally with an intensity of 60% for 10 s, according to information given by the manufacturer. Irradiation was done twice a week (once every third day) for a maximum of 10 sessions (Fig. 2). When the tip of the probe is placed in contact with the body, it emits energy around the treated area and splits environmental diatomic oxygen into singular atomic oxygen and ozone. The concentration of ozone in the operation field is 10~100 µg/ml.
Patients in the positive control group were treated with local corticosteroids consisting of dexamethasone mouthwash (Deksamet syrup; Osel Drug, Istanbul, Turkey) for 5 min, followed 30 min later by a mouth rinse with 30 drops of nystatin solution (100,000 units) (Mycostatin oral suspension; Deva Drug, Istanbul, Turkey) (four times a day for 1 month). The patients were followed weekly during this period.
A special solution filled with base ointment without the active corticosteroid component was prepared for patients in the negative control group, such as a dexamethasone mouthwash package. The patients gargled with this solution for 5 min. This application was repeated four times a day for 1 month.
The ozone and laser therapies were performed by one operator (H.O.K.). Another skilled examiner (M.E.), who was blind to the treatment received by each patient, recorded the group and size of the lesion and the patient-reported pain.
The means of patient's records, oral investigation, symptom changes, and clinical signs were determined for each patient. Erosive-atrophic OLP consists of reticular, erosive, and atrophic lesions at the same time. The severity of the lesions was scored according to presence of reticular, atrophic, or erosive lesions as follows: reticular/hyperkeratotic lesions were scored from 0 to 1 (0=no white striations, 1=appearance of white striations); erosive/erythematous areas were scored from 0 to 3 according to area of involvement (0=no lesion, 1=lesions <1 cm2, 2=lesions from 1 to 3 cm2, 3=lesions >3 cm2); and ulcerative areas were scored from 0 to 3 according to area of involvement (0=no lesion, 1=lesions <1 cm2, 2=lesions from 1 to 3 cm2, 3=lesions >3 cm2). For patients with more than one OLP lesion, a sign score was derived. The mouth was divided into four areas (right and left buccal mucosa, and right and left border of the tongue), and the scoring system was arranged with the summation of the scores of all four areas [reticular score=R, atrophic score=A, erosive/ulcerative score E (RAE score), with a total weighted score of R+(A×1.5)+(E×2.0)]18.
Reduction in the sign scores were scored according to the sign scoring scale of Thongprasom et al.19, as follows: 5 (white striae with an erosive area >1 cm2), 4 (white striae with an erosive area <1 cm2), 3 (white striae with an atrophic area >1 cm2), 2 (white striae with an atrophic area <1 cm2), 1 (mild white striae only), and 0 (no lesions, normal mucosa). The difference between baseline and after-treatment scores numerically expresses the clinical and symptomatic improvement.
The efficacy indices (EI) of the LLLT, ozone, and corticosteroid treatments were calculated on a five-rank scale according to the methods of Liu et al.20, with some modifications as follows: [(before treatment total score after treatment total score)÷before treatment total score]×100%. According to this scoring system, symptom improvement was scored as follows: healed, EI=100%; marked improvement, 75%≤EI<100%; moderate improvement, 25%≤EI<75%; mild improvement, 25%<EI<0; no improvement=0.
To evaluate the pain experience of the patients, a 0 to 10 visual analogue scale (VAS) was used, with the following scores: 3 (7<VAS≤10), 2 (3.5<VAS≤7), 1 (0<VAS≤3.5), and 0 (no pain)21.
Two oral medicine clinicians who were not authors of the current study evaluated each subject weekly for the improvement rate and adverse effects during treatment. The lesions of the patients were followed at 1, 3, and 6 months after the treatment to evaluate any residue, recurrence, or change. Data were analyzed by using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics including mean, median, and standard deviation were used. Also, inferential statistics including the chi-square, Mann-Whitney, Student's t, and Fisher's exact tests were used. The Wilcoxon sign test was used to evaluate the difference in the sign score of lesions between before and after treatment. Values of p<0.05 were considered statistically significant.
The study consisted of 120 OLP (56 male and 64 female) patients with a combined mean age of 42.6±8.3 years (range, 28~55 years). No statistically significant difference was detected in sex distribution, location, and clinical severity of the lesions among groups.
The Wilcoxon sign test showed that the sign scores decreased in almost all scoring groups of the LLLT-, ozone-, and corticosteroid-treated patients; however, a statistically significant improvement was found in the ozonated and corticosteroid-treated groups (Fig. 3). No significant difference was found among the groups according to symptom improvement before and after treatments. Symptom improvement and pain increment were achieved after treatment in corticosteroid- and ozone-treated patients (p<0.05) (Fig. 4, 5, Table 1). The EI were significantly higher in the ozonated and corticosteroid-treated groups (Table 2). No statistically significant association was detected between the location of lesions and response to treatment (p=0.631). There were no serious intra- or postoperative complications, and we have not seen any other adverse effects during the follow-up period.
After the cessation of topical treatment with corticosteroid ointment, 15 of the 30 patients (50%) experienced a relapse of OLP within 4~17 weeks; the mean time to relapse was 5 weeks. In ozonated group, 12 of the 30 patients (40%) had a relapse within 3.5~16 weeks; the mean time to relapse was 4.3 weeks.
There are various treatment strategies for OLP. Topical corticosteroids are recommended because OLP is an immunologically mediated condition, and intralesional and systemic corticosteroids are also used. Other medications include immunosuppressants (e.g., cyclosporine and tacrolimus), topical or systemic retinoids, and oral metronidazole8.
Use of local or systemic corticosteroids and nonsteroidal anti-inflammatory drugs may manifest several adverse effects. Therefore, a new nonmedication treatment that is comfortable to patients is necessary.
Laser surgery for oral leukoplakia was first performed by Ben-Bassat et al.22 Owing to the numerous advantages of the laser, for example, its hemostatic effect, limited scarring and cicatricial contractions, minimal thermal damage to adjacent tissues, and minimal postoperative pain, it has since been used for the treatment of oral mucosal lesions by many researchers23.
Unlike high-power lasers used to break down tissue thermally, LLLT is considered to work through the interaction of light with the cell and tissue. This interaction might be affected by some parameters such as wavelength, power, energy density, treatment duration and intervention time, method of application, structure, and condition of tissue. The dose of laser applied is an important treatment benefit of LLLT. However, a precisely determined dose has not been proved for each indication. Biostimulation has been reported in the literature with doses between 0.001 and 10 J/cm2 as a therapeutic window23. Although the applied dose is in the therapeutic window range, it might be too low or too high for the desired effect. Mester et al.24 reported that doses of ~1~2 J/cm2 are necessary to see an effect on wound healing.
The diode laser is currently used in many medical fields; however, only a few reports discuss its use in treating lesions of the oral mucosa, and OLP in particular2526272829. The results of earlier studies that used excimer lasers for the treatment of oral mucosal lesions were disappointing. Passeron et al.27 used excimer lasers (308 nm) for the treatment of four OLP patients, and no improvement was observed in three patients. The laser allowed only a stabilization of the OLP lesions in two of them. In the last patient, a worsening of the disease was noted. Köllner et al.28 also studied the effect of excimer lasers on eight patients with OLP, and only one patient responded to the treatment completely. Trehan and Taylor26 used an excimer laser in eight patients with OLP who had previously failed to respond to traditional treatment. Of the eight patients, five showed >75% improvement. In the current study, a GaAlAs laser with a wavelength of 808 nm, output power of 10 mW/cm2, and 4 J/cm2 energy density was used. This dose has also been proved to enhance epithelialization and wound healing by previous studies2530.
In this study, the improvement in terms of sign score, symptoms, and efficacy index scores is significantly greater in the ozone- and corticosteroid-treated groups than in the LLLT-treated and negative control groups. In contrast to the current study findings, Jajarm et al.25 reported that treatment of OLP with similar laser parameters was as effective as topical corticosteroid therapy. This difference could be because of the small sample size in their study.
Another physical stimulus method used for wound and bone healing is ozone therapy. Ozone (three atoms of oxygen instead of two) is normally present as a gas. The healing properties of ozone therapy resemble the biostimulatory property of LLLT that has been widely studied for the treatment of OLP2526. On the basis of this finding, some authors suggest that ozone therapy may be useful in the management of bone necrosis or in extractive sites during and after oral surgery in patients treated with bisphosphonates because it might stimulate cell proliferation and soft tissue healing31. Ozdemir et al16. analyzed the effect of ozone therapy in combination with autogenous bone grafts on bone healing in critical-size defect in rat calvaria, and they reported that ozone therapy increased bone formation.
Application of gaseous ozone has also been shown to be effective in facilitating oral wound healing after high-dose radiotherapy. Agrillo et al.31 and Petrucci et al.32 reported that ozone is effective when used 7 days before and after tooth extraction in patients with avascular bisphosphonate-related jaw osteonecrosis. Contrarily, Matsumura et al.33 reported that ozone does not have a major impact on the stimulation of gingival cells for osteoblastic activity in the regeneration of the periodontium around implants.
In the current clinical trial, the efficacy of two new procedures, LLLT and ozone therapies, in the treatment of the OLP was evaluated. The results of this study show that ozone therapy has a significant beneficial effect in controlling the signs of OLP, with no adverse effects. However, the positive effect of LLLT therapy in controlling the signs of OLP is not significant. Each of the treatment models has a positive effect in the control of the main symptoms of OLP. The size of the OLP lesions significantly decreased in the side treated with ozone therapy. No scar formation was observed after the ozone and laser treatments.
In the current study, the pain score had a statistically significant improvement in the corticosteroid- and ozone-treated groups. Most of the patients reported immediate pain relief after the first application of the treatments, and the majority of them reported a complete resolution of symptoms at the end of the treatment. As one of the primary end points in OLP treatment is to control the symptoms, this point is especially remarkable. Current treatments may reduce the pain and severity of lesions but do not actually cure OLP.
In the current study, 60% of patients had >50% improvement of their lesions in the group treated with ozone therapy, and 55% of patients had >45% improvement of their lesions in the group treated with corticosteroid therapy; this ratio decreased to 25% in the LLLT group. Four female patients with severe psychological problems (two patients in the LLLT group and two patients in the ozone therapy group) had no change in their lesions. This situation supports the hypothesis by Koray et al.34 that stress is the most frequent cause of OLP.
Although the sample size of this study was small, it seems that ozone, laser, and corticosteroid therapies have a beneficial effect on the treatment of OLP symptoms. In comparing the groups, however, ozone and corticosteroid therapies were found to be more effective than 808-nm LLLT. In addition, ozone and laser therapies did not exhibit unwanted adverse effects such as candida overgrowth. On the basis of the findings of the current study, ozone therapy could be considered a sufficient replacement to steroid treatment. Nevertheless, more detailed randomized controlled trials are needed.
Figures and Tables
Fig. 3
Mean sign scores of the groups at baseline and 1, 3, and 6 months after the end of treatments (Wilcoxon rank sum test: significant; p<0.05). LLLT: low-level laser therapy.

ACKNOWLEDGMENT
The authors thank S. Delacroix for improving the English of the manuscript and Dr. Omer Uysal for his expertise in conducting the statistical analysis.
References
1. Scully C, Carrozzo M. Oral mucosal disease: lichen planus. Br J Oral Maxillofac Surg. 2008; 46:15–21.

2. Axéll T, Zain RB, Siwamogstham P, Tantiniran D, Thampipit J. Prevalence of oral soft tissue lesions in out-patients at two Malaysian and Thai dental schools. Community Dent Oral Epidemiol. 1990; 18:95–99.

3. Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, et al. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis. 2009; 15:235–243.

4. Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med. 1998; 9:86–122.

5. Carrozzo M, Francia Di Celle P, Gandolfo S, Carbone M, Conrotto D, Fasano ME, et al. Increased frequency of HLA-DR6 allele in Italian patients with hepatitis C virus-associated oral lichen planus. Br J Dermatol. 2001; 144:803–808.

6. Bez C, Hallett R, Carrozzo M, Lodi G, Gandolfo S, Carrassi A, et al. Lack of association between hepatotropic transfusion transmitted virus infection and oral lichen planus in British and Italian populations. Br J Dermatol. 2001; 145:990–993.

7. Rubaci AH, Kazancioglu HO, Olgac V, Ak G. The roles of matrix metalloproteinases-2, -7, -10 and tissue inhibitor of metalloproteinase-1 in the pathogenesis of oral lichen planus. J Oral Pathol Med. 2012; 41:689–696.

8. Lozada-Nur F, Miranda C. Oral lichen planus: topical and systemic therapy. Semin Cutan Med Surg. 1997; 16:295–300.

9. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 82:644–649.

10. López-Ramírez M, Vílchez-Pérez MA, Gargallo-Albiol J, Arnabat-Domínguez J, Gay-Escoda C. Efficacy of low-level laser therapy in the management of pain, facial swelling, and postoperative trismus after a lower third molar extraction. A preliminary study. Lasers Med Sci. 2012; 27:559–566.

11. Mete O, Keskin Y, Hafiz G, Kayhan KB, Unur M. Oral proliferative verrucous leukoplakia: underdiagnosed oral precursor lesion that requires retrospective clinicopathological correlation. Dermatol Online J. 2010; 16:6.

12. Cianfriglia F, Di Gregorio DA, Cianfriglia C, Marandino F, Perrone Donnorso R, Vocaturo A. Incidence of human papillomavirus infection in oral leukoplakia. Indications for a viral aetiology. J Exp Clin Cancer Res. 2006; 25:21–28.
13. Cafaro A, Arduino PG, Massolini G, Romagnoli E, Broccoletti R. Clinical evaluation of the efficiency of low-level laser therapy for oral lichen planus: a prospective case series. Lasers Med Sci. 2014; 29:185–190.

14. Cafaro A, Albanese G, Arduino PG, Mario C, Massolini G, Mozzati M, et al. Effect of low-level laser irradiation on unresponsive oral lichen planus: early preliminary results in 13 patients. Photomed Laser Surg. 2010; 28:Suppl 2. S99–S103.

15. Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008; 9:75–84.

16. Ozdemir H, Toker H, Balcı H, Ozer H. Effect of ozone therapy on autogenous bone graft healing in calvarial defects: a histologic and histometric study in rats. J Periodontal Res. 2013; 48:722–726.

17. Ferrante M, Petrini M, Trentini P, Perfetti G, Spoto G. Effect of low-level laser therapy after extraction of impacted lower third molars. Lasers Med Sci. 2013; 28:845–849.

18. Piboonniyom SO, Treister N, Pitiphat W, Woo SB. Scoring system for monitoring oral lichenoid lesions: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99:696–703.

19. Thongprasom K, Luangjarmekorn L, Sererat T, Taweesap W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J Oral Pathol Med. 1992; 21:456–458.

20. Liu J, Zeng X, Chen Q, Cai Y, Chen F, Wang Y, et al. An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort: a randomized, double-blind, vehicle-controlled, unparallel multicenter clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:475–481.

21. Gorsky M, Raviv M. Efficacy of etretinate (Tigason) in symptomatic oral lichen planus. Oral Surg Oral Med Oral Pathol. 1992; 73:52–55.

22. Ben-Bassat M, Kaplan I, Shindel Y, Edlan A. The CO2 laser in surgery of the tongue. Br J Plast Surg. 1978; 31:155–156.

23. Ishii J, Fujita K, Komori T. Laser surgery as a treatment for oral leukoplakia. Oral Oncol. 2003; 39:759–769.

24. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971; 122:532–535.

25. Jajarm HH, Falaki F, Mahdavi O. A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus. Photomed Laser Surg. 2011; 29:421–425.

26. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004; 140:415–420.

27. Passeron T, Zakaria W, Ostovari N, Mantoux F, Lacour JP, Ortonne JP. Treatment of erosive oral lichen planus by the 308 nm excimer laser. Lasers Surg Med. 2004; 34:205.

28. Köllner K, Wimmershoff M, Landthaler M, Hohenleutner U. Treatment of oral lichen planus with the 308-nm UVB excimer laser--early preliminary results in eight patients. Lasers Surg Med. 2003; 33:158–160.

29. Yu CH, Lin HP, Chen HM, Yang H, Wang YP, Chiang CP. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg Med. 2009; 41:628–633.

30. Haker EH, Lundeberg TC. Lateral epicondylalgia: report of noneffective midlaser treatment. Arch Phys Med Rehabil. 1991; 72:984–988.
31. Agrillo A, Ungari C, Filiaci F, Priore P, Iannetti G. Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. J Craniofac Surg. 2007; 18:1071–1075.

32. Petrucci MT, Gallucci C, Agrillo A, Mustazza MC, Foà R. Role of ozone therapy in the treatment of osteonecrosis of the jaws in multiple myeloma patients. Haematologica. 2007; 92:1289–1290.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download