Abstract
Marjolin's ulcer is an aggressive cutaneous malignancy common in previously traumatized or chronically inflamed skin. It has high regional metastasis and fatality rates. Our patient presented with subcutaneous nodules and ulcerations on the right limb. He had a history of osteomyelitis of the fifth toe. Histopathological examination of the nodule and ulceration demonstrated squamous cell carcinoma. The nodules and ulcerations were in-transit metastases of Marjolin's ulcer. Here, we present a case of squamous cell carcinoma arising at a site of a chronic osteomyelitis with resultant in-transit metastases.
Marjolin's ulcer refers to a malignant transformation of chronic wounds; it is a rare but aggressive squamous cell carcinoma (SCC) that is most commonly associated with chronic burn wounds and chronic fistulae. It has also been reported with chronic osteomyelitis, venous and decubitus ulcers, hidradenitis suppurativa, vaccination scars, pilonidal sinus, skin graft donor sites, chronic pressure ulcers, and discoid lupus erythematosus12. Marjolin's ulcers are usually more aggressive than other primary SCCs and have higher regional metastasis and fatality rates.
In-transit metastasis is defined as intralymphatic metastasis more than 2 cm away from the primary tumor but before the first echelon of regional lymph nodes3. The concept of in-transit metastasis has been well described in melanoma, but in-transit metastasis in cutaneous SCC was only recently reported in organ transplant recipients4. It is associated with aggressive disease and a poor prognosis.
Here, we report a case of in-transit cutaneous metastases from a Marjolin's ulcer on the right leg.
A 53-year-old man presented with a 1-month history of multiple subcutaneous nodules and ulcerations over the right lower extremity and unilateral lymphedema. Regarding his medical history, he had a 6-year history of right fifth toe injury that was surgically repaired but had never healed. The toe had been amputated because of osteomyelitis 1 year previously. However, the ulceration had not been resolved despite long-term antibiotic therapy. In recent months, he developed right lower-extremity edema, nodules on the leg, and ulcerations on the dorsum of the right foot, with gradually increasing severity.
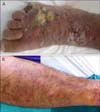
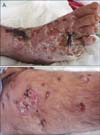
On dermatologic examination, he had an amputated fifth toe and 3 irregularly shaped deep ulcerations on the dorsum of the right foot, approximately 5 cm in diameter (Fig. 1A). He had multiple erythematous subcutaneous nodules all over his right lower extremity (Fig. 1B). During hospitalization, he developed 10~12 new ulcerations 0.5~1 cm in diameter on the right lower extremity (Fig. 2). As the right lower limb was edematous from the foot to the groin, lymph node palpation could not be performed. On magnetic resonance imaging, few sub-centimeter lymphadenopathies were observed.
Incisional biopsy was performed on a nodule and the margins of an ulceration for the differential diagnosis of sporotrichosis, actinomycosis, and atypical mycobacteria infections. Simultaneous tissue cultures revealed methicillinsensitive Staphylococcus aureus and Acinetobacter species. Therefore, intravenous ampicillin/sulbactam 1.5 g 4 times/day and oral ciprofloxacin 500 mg twice daily were administered. Meanwhile, ulcerations on the dorsum of the right foot gradually enlarged and became necrotic despite wound care with wet and antiseptic wound dressings as well as antibiotics. Myiasis developed on the 15th day of hospitalization. Screwworms were removed in 4 days by using irrigation with diluted alcohol solution.
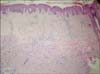
Histopathological examination of the specimens showed poorly differentiated SCC with deep invasion of the dermis and subcutaneous adipose tissue. There was no neurovascular invasion (Fig. 3). Laboratory evaluations including full blood counts, sedimentation, and biochemistry were normal. Cranial, thoracic, and abdominal computed tomography did not show any organ involvement. After consultation with the plastic surgery, orthopedic surgery, and medical oncology departments, systemic chemotherapy was suggested as first-line treatment, but the patient refused. Therefore, hemipelvectomy surgery was discussed with the patient as second-line treatment, but he refused this option as well. After he was discharged from the hospital, he did not attend follow-up appointments. He died 2 months later, but no information about the actual cause of death was available.
Marjolin's ulcer is a SCC that develops in posttraumatic scars and chronic wounds. However, a diagnosis of SCC with Marjolin's ulcer is uncommon. The incidence of chronic osteomyelitis developing into SCC ranges from 0.2%~1.7%5.
Most of these tumors are located on the extremities, particularly the lower limbs. Malignant transformation occurs after a mean of 43 years, ranging from 10 to 70 years. Tenopyr and Silverman6 state that at least 3 years is required for the progression of an ulcer to malignant transformation1. Although the malignant transformation of chronic wounds requires further clarification, various factors are implicated, including toxins released from damaged tissue, immunological factors, repeated irritation, poor lymphatic regeneration, co-carcinogens, DNA mutations, and local toxins7.
SCCs originating from these lesions are more aggressive than other primary SCCs. Marjolin's ulcer has a higher tendency for local recurrence and distant metastasis via the lymphatic system57. The most significant prognostic factors predicting recurrence are histological staging and tumor grade. Anatomic location also appears to play an important role in the metastatic potential of the tumor78. Tumors of the lower extremities, as in the present case, also have a higher risk of recurrence than tumors at other locations. The metastasis rate of lower-extremity lesions is reported to be 30%, whereas Novick et al.9 report an overall metastasis rate of 53.8%. Once metastasis has occurred, the mortality rate is also high: up to 32.6%8.
In-transit metastasis is frequently reported in malignant melanoma but is rarely reported in other primary cutaneous malignancies. The American Joint Committee on Cancer (AJCC) staging system for cutaneous melanoma defines intransit metastasis as, "intralymphatic metastasis occurring more than 2 cm from the primary tumor." In-transit metastasis is a poor prognostic feature in melanoma; like nodal involvement, it signifies stage III disease10. Reports of intransit metastases from primary cutaneous SCC are limited to single case reports and one multicenter study of 21 patients, 15 of whom were iatrogenically immunosuppressed organ-transplant recipients. Risk factors for the development of in-transit metastases are immunosuppressed status, large primary lesions, lesions on the head and neck, histologically poorly differentiated lesions, and recurrent tumors410.
The prognostic significance of in-transit metastases in SCC is less clear than that in melanoma. Intralymphatic metastasis is not mentioned in the current AJCC system for SCC11. Dinehart and Peterson12 recently pointed out some deficiencies of the current AJCC staging system for cutaneous SCC; they state that satellite and in-transit metastases, which are locally invasive tumors, are different from lymph node metastases and that the prognosis of a patient with a locally invasive tumor will never be similar to one with a nodal metastasis. Therefore, they proposed a new classification system1112. Additional studies in this area may elucidate the prognostic significance of in-transit metastasis in cutaneous SCC.
Epidermotropic and sporotrichoid cutaneous metastases from cutaneous SCC have been described in the literature. Clinically, in-transit metastases from cutaneous SCC are mostly subcutaneous or dermal papules with occasional exophytic features413. The 2 cases reported by Weidner and Foucar14 were SCC of the lower lip and hand that had in-transit metastases to the submental region of the skin and an upper extremity, respectively. Meanwhile, Copcu et al.13 report 2 cases of in-transit cutaneous metastasis with an acantholytic pattern from a skin tumor on the face. Wain et al.10 report a case of extensive cutaneous in-transit metastasis in an immunocompetent man with cutaneous SCC of the toe. In the present case, the patient had multiple subcutaneous nodules arising from the lymphedema in the right lower extremity. Therefore, our initial suspicion was sporotrichosis, actinomycosis, or atypical mycobacteria infection. However, new ulcers occurred rapidly, and histopathologic examination revealed SCC. Meanwhile, the patient had ulcers and subcutaneous nodules compatible with the definition of in-transit metastases. The lymphedema in our case may have been contributing to the development of in-transit metastases; a possible explanation for this is the increased extravasation of tumor cells within the lymphedematous limb and delayed lymphatic return.
Treatment options for multiple in-transit metastases include intralesional or systemic chemotherapy, radiotherapy, excision, amputation, and hyperthermic isolated limb perfusion1015. In particular, hyperthermic isolated limb perfusion is an isolated regional therapy that can control unresectable advanced local or in-transit disease, avoiding amputation and preserving limb functionality15. Single cases responding to cetuximab and topical miltefosine have also been reported1617.
Histopathologic examination is important, particularly in osteomyelitis patients with chronic ulcers, and SCC should be suspected in these cases. Clinicians should be aware that a Marjolin's ulcer is more aggressive and more prone to metastasis than other skin cancers of the same cell type. Delayed presentation or misdiagnosis can lead to systemic and potentially fatal metastasis. Therefore, a policy of low threshold to biopsy for chronic non-healing ulcers should be adopted; repeated biopsies may be necessary.
In summary, we report a case of extensive cutaneous intransit metastasis in a patient with a Marjolin's ulcer.
Figures and Tables
Fig. 1
(A) Irregularly shaped deep ulcerations on the dorsum of the right foot. (B) Multiple erythematous subcutaneous nodules on the right lower extremity.
References
1. Tavares E, Martinho G, Dores JA, Vera-Cruz F, Ferreira L. Marjolin's ulcer associated with ulceration and chronic osteomyelitis. An Bras Dermatol. 2011; 86:366–369.
2. Ashraf M, Biswas J. Chronic ringworm infestation and Marjolin's ulcer, an association unknown in the literature. Rare Tumors. 2010; 2:e31.

3. Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg. 2004; 91:673–682.

4. Carucci JA, Martinez JC, Zeitouni NC, Christenson L, Coldiron B, Zweibel S, et al. In-transit metastasis from primary cutaneous squamous cell carcinoma in organ transplant recipients and nonimmunosuppressed patients: clinical characteristics, management, and outcome in a series of 21 patients. Dermatol Surg. 2004; 30:651–655.

5. Ogawa B, Chen M, Margolis J, Schiller FJ, Schnall SB. Marjolin's ulcer arising at the elbow: a case report and literature review. Hand (N Y). 2006; 1:89–93.

6. Tenopyr J, Silverman I. The relation of chronic varicose ulcer to epithelioma. Ann Surg. 1932; 95:754–758.

7. Copcu E, Aktas A, Sişman N, Oztan Y. Thirty-one cases of Marjolin's ulcer. Clin Exp Dermatol. 2003; 28:138–141.

8. Sabin SR, Goldstein G, Rosenthal HG, Haynes KK. Aggressive squamous cell carcinoma originating as a Marjolin's ulcer. Dermatol Surg. 2004; 30:229–230.

9. Novick M, Gard DA, Hardy SB, Spira M. Burn scar carcinoma: a review and analysis of 46 cases. J Trauma. 1977; 17:809–817.
10. Wain EM, Webber NK, Stefanato CM, Banerjee P, Morris SL. Multiple in-transit cutaneous metastases from a primary cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2009; 34:522–524.

11. Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007; 33:885–899.

12. Dinehart SM, Peterson S. Evaluation of the American Joint Committee on cancer staging system for cutaneous squamous cell carcinoma and proposal of a new staging system. Dermatol Surg. 2005; 31:1379–1384.

13. Copcu E, Dikicioglu E, Sivrioglu N, Meteoglu I. Subcutaneous nodules on the face: acantholytic in-transit cutaneous metastasis. Dermatol Surg. 2004; 30:1415–1419.

14. Weidner N, Foucar E. Epidermotropic metastatic squamous cell carcinoma. Report of two cases showing histologic continuity between epidermis and metastasis. Arch Dermatol. 1985; 121:1041–1043.

15. Olieman AF, Liénard D, Eggermont AM, Kroon BB, Lejeune FJ, Hoekstra HJ, et al. Hyperthermic isolated limb perfusion with tumor necrosis factor alpha, interferon gamma, and melphalan for locally advanced nonmelanoma skin tumors of the extremities: a multicenter study. Arch Surg. 1999; 134:303–307.

16. Bauman JE, Eaton KD, Martins RG. Treatment of recurrent squamous cell carcinoma of the skin with cetuximab. Arch Dermatol. 2007; 143:889–892.

17. Mahieu-Renard L, Richard MA, Dales JP, Buscaylet S, Lagrassa S, Grob JJ. Treatment of cutaneous metastases of a squamous cell carcinoma of the leg with topical miltefosine. Ann Dermatol Venereol. 2005; 132:346–348.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download