Abstract
Background
Acne is an inflammatory skin disorder caused by inflammatory biomarkers. Magnesium ascorbyl phosphate (MAP) is a stable precursor of vitamin C. It achieves a constant delivery of vitamin C into the skin and has antioxidative effects.
Objective
We performed this study to evaluate the effect of MAP on the expression of inflammatory biomarkers in cultured sebocytes.
Methods
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay were performed for inflammatory cytokines and matrix metalloproteinases (MMPs) before and after treatment of cultured sebocytes with MAP (10-2 M), lipopolysaccharide (LPS) (5 µg/ml) and a combination of MAP and LPS. RT-PCR and western blotting were also performed for antimicrobial peptides (AMPs) and Toll-like receptor (TLR)-4 before and after treatment of cultured sebocytes with MAP, LPS, and a combination of MAP and LPS. Quantification of lipid peroxidation was also conducted.
Results
The increased expression of inflammatory cytokines after treatment of cultured sebocytes with LPS was decreased after treatment with MAP. MMPs, AMPs, and TLR-4 were decreased after treatment of cultured sebocytes with MAP and a combination of MAP and LPS, and increased after treatment of cultured sebocytes with LPS alone. Lipid peroxidation was significantly decreased after treatment of cultured sebocytes with MAP and a combination of MAP and LPS. MAP decreased the increased lipid peroxidation after treatment of cultured sebocytes with LPS.
Acne is a common skin disorder in adolescence and adult. Pathogenic factors of acne include abnormal folliculoinfundibular keratinization, Propionibacterium acnes proliferation, and effects of androgen hormone. In addition, sebum secretion from the sebaceous gland also plays an important role in the pathophysiology of acne1. Excessive sebum production, abnormal sebum composition, sebum peroxidation, and proinflammatory lipid production all contribute to the formation of the primary acne lesions2. Furthermore, sebaceous glands also produce inflammatory cytokines and antimicrobial peptides (AMPs), which also play a vital role in the formation and aggravation of acne lesions3. Production of proinflammatory cytokines is induced by the activation of nuclear factor kappa B (NF-κB) through the Toll-like receptor (TLR)-2 or TLR-4. Lipoteichoic acid from the gram-positive P. acnes binds to TLR-2 and lipopolysaccharide (LPS) from gram-negative bacteria bind to TLR-4.
Vitamin C contains L-ascorbic acid, calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate (MAP), sodium ascorbate, and sodium ascorbyl phosphate. Vitamin C is associated with several beneficial properties, including the promotion of collagen synthesis, photoprotection from ultraviolet A and B radiation, lightening of hyperpigmentation, and improvement of a variety of inflammatory dermatoses4. It also has antioxidant properties and is effective in treating acne and acne scarring. MAP is a stable vitamin C derivative; as a powerful antioxidant, it has anti-inflammatory effects and prevents sebum oxidation in acne vulgaris.
This study was performed to evaluate whether the expression of inflammatory cytokines, matrix metalloproteinases (MMPs), AMPs, and TLR-4 after treatment of cultured sebocytes with MAP, LPS, and a combination of MAP and LPS is decreased. Whether sebum peroxidation decreases was also evaluated under the same conditions.
Primary sebocyte cultures were obtained from occipital hair follicle sebaceous glands. From the hair follicles, the sebaceous glands were dissected under a binocular microscope and transferred to a tissue culture dish. The cells were maintained in Dulbecco's modified Eagle medium (DMEM; Hyclone Laboratories, Logan, UT, USA) at 37℃ in a humidified atmosphere of 5% CO2. Explants were incubated for three days, and the medium was then changed to Epilife (MEPI500CA; Gibco BRL, Grand Island, NY, USA). The medium was changed every three days. DMEM was supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), and 20% heat inactivated bovine serum (Hyclone Laboratories), while Epilife was supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), and fungizone (250 µg/ml).
Once the cells became subconfluent, they were harvested using 0.25% trypsin and 10 mM ethylenediamine tetraacetic acid (EDTA) in Hank's balanced salt solution, followed by subculturing at a split ratio of 1:3. Cells obtained after the second passage were used in this study. These cultured sebocytes were subjected to hematoxylin and eosin (Muto Pure Chemicals Co., Tokyo, Japan) and Oil Red O (Sigma, St. Louis, MO, USA) staining, and immunocytofluorescence against cytokeratin 1 and 7 (Chemicon, Billerica, MA, USA) before experimental use (Fig. 1).
MAP is a stable vitamin C precursor that achieves constant delivery of vitamin C into the skin. The cultured sebocytes were treated for 24 h with MAP (10-2 M) (Sigma-Aldrich, St. Louis, MO, USA), LPS from Escherichia coli (5 µg/ml) (Sigma-Aldrich) or a combination of MAP (10-2 M) and LPS from E. coli (5 µg/ml). The concentration of MAP used was determined by an MTT assay.
RNA was reverse transcribed using a first strand cDNA synthesis kit (Promega, Madison, WI, USA). PCR amplification was conducted in triplicate with oligonucleotide primers (Genotech, Daejeon, Korea) for β-actin (5'-GGG AAA TCG TGC GTG ACA TT, 5'-GGA GTT GAA GGT AGT TTC GTG), interleukin (IL)-1β (5'-GGG CCT CAA GGA AAA GAA TC, 5'-TTC TGC TTG AGA GGT GCT GA), IL-6 (5'-TAC CCC CAG GAG AAG ATT CC, 5'-GAG GTG CCC ATG CTA CAT TT), IL-8 (5'-AGA TAT TGC ACG GGA GAA, 5'-GAA ATA AAG GAG AAA CCA), tumor necrosis factor (TNF)-α (5'-TCC TTC AGA CAC CCT CAA CC, 5'-GGC TAC ATG GGA ACA GCC TA), MMP-9 (5'-CAC TGT CCA CCC CTC AGA GC, 5'-GCC ACT TGT CGG CGA TAA GG), LL-37 (5'-GGG TAG GGC ACA CAC TAG GA, 5'-GGA CAG TGA CCC TCA ACC AG) and TLR-4 (5'-CCA TGT TCA TTG TGG CAC TC, 5'-TCC CTT CCT CCT TTT CCC TA). Image J (NIH Image, Bethesda, MD, USA) was used to quantify relative expression levels. mRNA levels were normalized to β-actin and represented as relative ratios. ANOVA was used for the statistical analysis of all data. A probability value of less than 0.05 was considered statistically significant.
TRIzol reagent (Invitrogen, Grand Island, NY, USA) with a modified acid phenol method was used to isolate total RNA from the cells.
cDNA was synthesized from 5 µg total RNA using a cDNA synthesis kit containing ImProm-II reverse transcriptase and random primers (Promega). GoTaq Flexi DNA Polymerase was used for PCR amplification. LL-37 amplifications were performed for 28 cycles at annealing temperature of 61℃. TLR-4 amplifications were performed for 35 cycles at annealing temperature of 56℃. All other amplifications were performed for 35 cycles under the following conditions: 95℃ for 1 min, 56℃ for 1 min, and 72℃ for 1 min, with the exception of IL-1β, which was performed for 25 cycles under the same program.
IL-1β, IL-6, IL-8, TNF-α, and MMP-9 (R&D Systems, Shanghai, China) protein expression was assessed by ELISA according to the manufacturer's instructions. Briefly, 50 µl of the sample was added to each well in triplicate. Next, 200 µl of prepared streptavidin-horseradish peroxidase and 200 µl of premixed 3,3',5,5'-tetramethylbenzidine substrate solution were added to each well in that order. The plates were developed in the dark at room temperature for 30 min, and the reaction was stopped by adding 50 µl of stop solution to each well. Finally, absorbance was measured on a VERSA maxmicroplate reader (Molecular Devices, Sunnyvale, CA, USA). ANOVA was used for statistical analysis of the data. A probability value of less than 0.05 was considered statistically significant.
Cultured sebocytes before and after treatment with MAP, LPS, and a combination of MAP and LPS were lysed in a lysis buffer (25 mM Tris-HCl [pH 7.2], 150 mM KCl, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 5 mM EDTA [pH 8.0], 2 mM phenylmethylsulfonyl fluoride) supplemented with protease inhibitor cocktail. Equal protein amounts of total cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and blotted with antibodies against LL-37 (Abcam, Cambridge, UK) and TLR-4 (Abnova, Jhongli City, Taiwan), followed by enhanced chemiluminescence and autoradiography. This procedure was performed in triplicate. ANOVA was used for statistical analysis of the data. A probability value of less than 0.05 was considered statistically significant.
BioVision lipid peroxidation assay kit (Biovision, Milpitas, CA, USA) was used according to the manufacturer's instructions for the sensitive detection of malondialdehyde assays. Sebocytes (1×106 treated with MAP, LPS, or a combination of MAP and LPS were homogenized on ice in 300 µl of MDA Lysis Buffer with 3 µl butylated hydroxytoluene (100×) and then centrifuged (13,000g, 10 min) to remove the insoluble material. The 200 µl supernatant from each homogenized sample and standard was placed into a microcentrifuge tube and 600 µl of thiobarbituric acid solution was added. The mixture was incubated at 95℃ for 60 min and then cooled in ice bath. For the standard curve, 0, 2, 4, 6, 8, and 10 µl of the 2 mM MDA Standard were added into separate microcentrifuge tubes. Then, 200 µl of each homogenized sample and standard were placed into a 96-well microplate. Absorbance was read at 532 nm.
Inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α are produced in unstressed cultured sebocytes. Their expression is influenced by many factors. In this study, LPS upregulated the gene and protein expression of the inflammatory cytokines in cultured sebocytes (Fig. 2, 3). MAP treatment of cultured sebocytes induced a significant decrease in IL-1β gene and protein expression when compared with the untreated control (p<0.05) (Fig. 2, 3). The LPS-induced increase in IL-1β gene and protein expression decreased significantly after treatment with MAP (p<0.05) (Fig. 2, 3). The LPS-induced increase in IL-6 protein expression in cultured sebocytes was inhibited by treatment with MAP (Fig. 3). The increased IL-8 gene expression after treatment with LPS was significantly decreased by treatment with MAP (p<0.05) (Fig. 2). In addition, increase in TNF-α gene and protein expression after treatment with LPS was inhibited by treatment with MAP (Fig. 2, 3).
Binding of LPS to TLR-4 may lead to increased expression of inflammatory cytokines. The treatment of cultured sebocytes with MAP decreased TLR-4 gene and protein expression when compared with an untreated control (Fig. 2, 3). In addition, an increase in TLR-4 gene and protein expression after treatment with LPS was decreased by treatment with MAP (Fig. 2, 3).
The treatment of cultured sebocytes with MAP showed a decrease in the gene and protein expression of MMP-9 when compared with untreated control (Fig. 2, 3). The increased gene and protein expression of MMP-9 in cultured sebocytes after treatment with LPS was decreased by treatment with MAP (Fig. 2, 3).
LPS, which are constituents of gram-negative bacteria, have been implicated as bacterial products responsible for inflammatory reactions through the induction of inflammatory mediators, such as cytokines (IL-1β, IL-8, TNF-α), MMPs, and AMPs in keratinocytes, sebocytes, dermal fibroblasts, and infiltrated immune cells567891011. Gram-negative bacteria have been reported in the skin lesions of acne patients and are associated with development of acne along with P. acnes61213.
Sebocytes are considered to play an important role in the pathogenesis of inflammatory acne through not only sebum production and changes in sebum composition but also the production of inflammatory mediators. Sebocytes respond to microbial products such as LPS through the activation of TLRs, especially TLR-4, and produce various cytokines that may evoke an immune response in inflammatory acne. In addition, sebocytes can induce prostaglandins, MMPs, and AMPs.
Pharmacologically active vitamin C has antioxidant and anti-inflammatory effects. However, vitamin C is very unstable under normal environmental conditions. MAP is a stable vitamin C compound that can attenuate the production of inflammatory mediators. It has not been reported whether MAP influences the inhibition of inflammatory mediators induced by LPS in cultured sebocytes. Therefore, we assessed the effect of MAP on the responses of sebocytes treated with LPS. In this study, MAP inhibited the production of IL-1β, IL-8, and TNF-α in cultured sebocytes after treatment with LPS. IL-1β is considered an important marker of inflammation in acne. IL-8 is known to act as a neutrophilic chemotactic factor in inflammation. TNF-α induces the production of cytokines, chemokines, and reactive oxygen species in keratinocytes through the activation of transcription factor NF-κB14.
Inflammatory reactions in acne have been reported to include extracellular matrix remodeling mediated by MMPs6151617. MMP isoforms have been reported in sebum, derived from sebocytes16. Furthermore, sebocytes were found to spontaneously express TLR-4. Expression of MMP-9 increased following treatment of cultured sebocytes with LPS, which was subsequently decreased by MAP treatment. Cathelicidin is also detected in cultured human sebocytes, and its expression levels are upregulated in the presence of P. acnes9. The LPS-induced increased expression of LL-37 in cultured sebocytes was decreased by MAP.
Similarly, the increased expression of TLR-4 with LPS treatment was decreased by MAP. The TLR-4 pathway may be inhibited by MAP. LPS-induced stimulation of the TLR-4 pathway and subsequent increase in expression of inflammatory cytokines, MMPs, and AMPs in cultured sebocytes warrant further evaluation of TLR-4-related transcription factors.
Akitomo et al.18 reported that treatment of cultured sebocytes with LPS facilitates the peroxidation of sebum lipids. Vitamin C has antioxidative properties. MAP is effective for the prevention of sebum peroxidation on the skin because of its own antioxidative activity. In this study, increased sebum peroxidation after treatment of cultured sebocytes with LPS was decreased by MAP.
In conclusion, this study showed that MAP as vitamin C may be an effective anti-inflammatory and antioxidant factor in cultured sebocytes. Thus, we propose that vitamin C should be considered as a supplementary material to treat inflammatory acne.
Figures and Tables
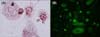 | Fig. 1Identification of cultured sebocytes by (A) oil red O stain (×1,000) and (B) immunohistochemistry against cytokeratin 7 (×400). |
 | Fig. 2Gene expression of interleukin (IL)-1β, IL-8, tumor necrosis factor (TNF)-α, matrix metalloproteinase (MMP)-9, LL-37, and Toll-like receptor (TLR)-4 was decreased after treatment of cultured sebocytes with magnesium ascorbyl phosphate (MAP) (10-2 M) when compared with an untreated control. The increased gene expression of IL-1β, IL-8, TNF-α, MMP-9, LL-37, and TLR-4 after treatment of cultured sebocytes with lipopolysaccharide (LPS) (5 µg/ml) was decreased after treatment of cultured sebocytes with MAP (10-2 M)+LPS (5 µg/ml) (*p<0.05). |
 | Fig. 3Protein expression of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and matrix metalloproteinase (MMP)-9 by enzyme-linked immunosorbent assay (ELISA) and that of LL-37 and Toll-like receptor (TLR)-4 by western blot was decreased after treatment of cultured sebocytes with magnesium ascorbyl phosphate (MAP) (10-2 M) when compared with an untreated control. The increased protein expression of IL-1β, IL-6, IL-8, TNF-α, and MMP-9 by ELISA and that of LL-37 and TLR-4 by western blot after treatment of cultured sebocytes with lipopolysaccharide (LPS) (5 µg/ml) was decreased after treatment of cultured sebocytes with MAP (10-2 M)+LPS (5 µg/ml) (*p<0.05). |
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A1A2007017); grant of Amore-Pacific Corporation, 2014.
References
1. Winston MH, Shalita AR. Acne vulgaris. Pathogenesis and treatment. Pediatr Clin North Am. 1991; 38:889–903.

3. Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Human skin stem cells and the ageing process. Exp Gerontol. 2008; 43:986–997.

4. Park YK, Chung WS, Lee H, Jung SW. Whitening effect of cosmetics containing magnesium l-ascorbyl-2-phosphate (VC-PMG, vitamin C derivatives) assessed by colorimeter. Ann Dermatol. 2002; 14:63–70.

5. Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl). 2006; 84:75–87.

6. Corbel M, Theret N, Caulet-Maugendre S, Germain N, Lagente V, Clement B, et al. Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm Res. 2001; 50:129–135.

7. Iwata C, Akimoto N, Sato T, Morokuma Y, Ito A. Augmentation of lipogenesis by 15-deoxy-Delta12,14-prostaglandin J2 in hamster sebaceous glands: identification of cytochrome P-450-mediated 15-deoxy-Delta12,14-prostaglandin J2 production. J Invest Dermatol. 2005; 125:865–872.

8. Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005; 211:193–198.

9. Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006; 8:2195–2205.

10. Oeff MK, Seltmann H, Hiroi N, Nastos A, Makrantonaki E, Bornstein SR, et al. Differential regulation of Toll-like receptor and CD14 pathways by retinoids and corticosteroids in human sebocytes. Dermatology. 2006; 213:266.

11. Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res. 2005; 11:5–11.

13. Böni R, Nehrhoff B. Treatment of gram-negative folliculitis in patients with acne. Am J Clin Dermatol. 2003; 4:273–276.

14. Zhou BR, Zhang JA, Zhang Q, Permatasari F, Xu Y, Wu D, et al. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediators Inflamm. 2013; 2013:530429.
15. Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen. 1999; 7:166–171.

16. Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005; 125:673–684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download