Abstract
Background
Keratinocytes release various pro-inflammatory cytokines, chemokines, and adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) in response to cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ. Rapamycin and mycophenolic acid (MPA) have potent immunosuppressive activity because they inhibit lymphocyte proliferation.
Objective
We investigated the effects of rapamycin and MPA on the expression of inflammation-related factors such as ICAM-1 and inducible nitric oxide synthase (iNOS), pro-inflammatory cytokines and chemokines, and related signaling pathways in TNF-α-stimulated HaCaT cells.
Methods
The viability of HaCaT cells treated with rapamycin and MPA was confirmed using MTT assay. The expression of various cytokines such as interleukin (IL)-1β, IL-6, and IL-8; inflammation-related factors such as ICAM-1 and iNOS; and the activation of mitogen activated protein kinase (MAPK) signaling pathways mediated by extracellular signal-related kinases (ERK), p38, and c-Jun N-terminal kinases (JNK) in TNF-α-stimulated HaCaT cells were confirmed using reverse transcription-polymerase chain reaction and western blotting.
Results
Combined treatment of TNF-α-induced HaCaT cells with rapamycin and MPA decreased ICAM-1 and iNOS expression and ERK and p38 activation more than treatment with either drug alone. The most significant decrease was observed with a combination of rapamycin (80 nM) and MPA (20 nM). These results show that co-treatment with these agents has a synergistic anti-inflammatory effect by blocking the activation of the ERK/p38 MAPK signaling pathway and thus suppressing the TNF-α-induced expression of ICAM-1 and iNOS.
The primary function of keratinocytes, which comprise 95% of the human epidermis, is to maintain the biochemical and physical integrity of the skin. It is now well accepted that they also play an important role in the skin's immune system1,2,3. Keratinocytes express and release immunomodulatory mediators in response to ultraviolet light, allergens, haptens, microbiological agents, and cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ. The expression of various pro-inflammatory cytokines, chemokines, and adhesion molecules allow immune cells to enter the site of inflammation in the skin4,5,6. TNF-α, a major pro-inflammatory cytokine, is produced by multiple cell types in the skin, including keratinocytes. It acts as a multifunctional cytokine, regulating the production of pro-inflammatory cytokines and chemokines such as interleukin (IL)-1β, IL-6, and IL-8, as well as adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1). In the skin, TNF-α also modulates the initial stages of the response to inflammation and injury7,8,9,10. The stimulation of keratinocytes by TNF-α leads to the activation of various signaling pathways that involve caspases, nuclear factor-kappa B (NF-κB), and mitogen activated protein kinases (MAPKs), which subsequently increase the expression of inflammatory mediators11.
NF-κB is a protein transcription factor that is required for the transcription of a wide array of pro-inflammatory molecules that are thought to be important in the onset of apoptosis, in various autoimmune diseases, and in inflammation12,13. In the resting state, NF-κB dimers are inactive in the cytoplasm of cells and are associated with the NF-κB inhibitory protein (I-κB). Upon stimulation with agents such as TNF-α, the IκB-kinase (IKK) complex is activated and phosphorylates IκB, leading to the substrate's ubiquitination and subsequent degradation. The resulting free NF-κB is translocated to the nucleus where it can activate target genes by binding to regulatory elements in the target gene's promoter14,15,16.
Rapamycin and mycophenolic acid (MPA) have potent immunosuppressive characteristics because they inhibit lymphocyte proliferation. At the molecular level, these drugs share several mechanistic similarities with other immunosuppressive drugs17,18. Rapamycin, also known as sirolimus, is a macrocyclic triene antibiotic that was first isolated from Streptomyces hygroscopicus in the early 1970s. This drug forms an intracellular complex with FK506-binding protein 12 (FKBP12) and inhibits the activity of mammalian target of rapamycin (mTOR)19,20.
MPA was initially marketed as mycophenolate mofetil (MMF), which has better oral bioavailability compared to that of its active metabolite, MPA21. MPA is a potent non-competitive inhibitor of inosine-monophosphate-dehydrogenase, and thus affects lymphocytes function by disabling the purine biosynthesis pathway. Recently, MPA has been used as a steroid treatment in immune-mediated disorders including immunoglobulin (Ig) A and psoriasis22,23,24.
We investigated the effects of different combinations of immunosuppressive drugs on the expression of pro-inflammatory mediators and found that the combination of rapamycin and MPA had a synergistically greater anti-inflammatory effect compared to that of single-drug treatments.
Rapamycin and MPA were synthesized at IKSU Co., Ltd. (Seoul, Korea). Dulbecco's modified Eagle medium (DMEM), Dulbecco's phosphate-buffered saline (Dulbecco's PBS), antibiotic (penicillin, streptomycin), fetal bovine serum (FBS), and trypsin-EDTA were purchased from WelGENE Inc. (Daegu, Korea). Recombinant human TNF-α was purchased from R&D Systems (Minneapolis, MN, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), PD98059, and SP600125 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies specific to ICAM-1, phospho-extracellular signal-related kinases (ERK), phospho-IκBα, and lamin B were purchased from Cell Signaling Technology (Beverly, MA, USA). phosphor-c-Jun N-terminal kinases (JNK) and Phosphor-p38, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), and inducible nitric oxide synthase (iNOS) antibodies were obtained from BD Bioscience (San Jose, CA, USA). Antibodies specific to NF-κB p65 were obtained from Abcam (Cambridge, MA, USA). Secondary antibodies specific for anti-goat IgG, anti-mouse IgG, and anti-rabbit IgG were purchased from Vector Laboratories (Burlingame, CA, USA). ICAM-1, iNOS, IL-1β, IL-6, IL-8, and GAPDH oligonucleotide primers were obtained from Bioneer (Seoul, Korea).
The human keratinocyte cell line (HaCaT) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured at 37℃ in a humidified incubator containing 5% CO2 and 95% air in DMEM supplemented with 10% FBS (WelGENE Inc.) and antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin).
Total RNA was isolated from cells by using an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The RNA (2 µg) was transcribed into cDNA using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Otsu, Japan). The transcribed product was amplified by polymerase chain reaction (PCR) into a final volume of 50 µl by using the following sense and antisense primers (5'→3'): ICAM-1 sense, GGT GAC GCT GAA TGG GGT TCC; ICAM-1 antisense, GTC CTC ATG GTG GGG CTA TGA CTC; iNOS sense, TCC AAC CTG CAG GTC TTC GAT GC; iNOS antisense, GGA CCA GCC AAA TCC AGT CTG C; IL-1β sense, AAA CAG ATG AAG TGG TCC TTC CAG; IL-1β antisense, TGG AGA ACA CCA CTT GTT GCT CCA; IL-6 sense, AGA GTA GTG AGG AAC AAG CC; IL-6 antisense, TAC ATT TGC CGA AGA GCC CT; IL-8 sense, ACA TGA CTT CCA AGC TGG CCG; IL-8 antisense, TTT ATG AAT TCT CAG CCC TC; and GAPDH sense, CAT GGG GAA GGT GAA GGT C; GAPDH antisense, TGG ACT CCA CGA CGT ACT CA. Amplification was performed using Taq polymerase (Takara, Otsu, Japan). The products were electrophoresed for 30 min at 100 V on 1% agarose gel. Gels were visualized using a Molecular Imager Gel Doc XR imaging system (Bio-Rad Laboratories Inc., Hercules, CA, USA).
HaCaT cells were washed twice with ice-cold PBS and then lysed in RIPA buffer. The lysates were clarified by 30 min of centrifugation at 13,000 rpm at 4℃. Total protein in the cell extracts or in the cytoplasmic and nuclear extracts was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% polyacrylamide gel. The separated proteins were transferred to a PVDF membrane and then the membrane was blocked with blocking buffer (5% skim milk in Tris-buffered saline containing 0.5% Tween 20). Western blot analysis was performed by first incubating the membrane in target antibodies overnight at 4℃, and then incubating them with horseradish peroxidase-conjugated secondary antibody. Detection of immunoreactive bands was performed using a SuperSignal West Pico chemiluminescence substrate (PIERCE Biotechnology Inc., IL, USA). The values of the western blot were standardized with an internal control.
Data are presented as means±standard deviations. Data were evaluated using the Student's t-test. A p-value of <0.05 or <0.01 was considered statistically significant. The analysis was performed with Statistical Package for Social Sciences (version 12.0; SPSS Inc., Chicago, IL, USA).
Cell viability was measured at different concentrations of rapamycin, MPA, or a combination of the two drugs by using MTT assay in HaCaT cells. HaCaT cells were incubated with various concentrations (10, 100, 1,000, or 5,000 nM) of rapamycin or MPA for 24 h (Fig. 1A). Viability was not significantly altered by concentrations of rapamycin or MPA up to 1,000 nM. As shown Fig. 1B, the cells were also treated with a combination of different concentrations of rapamycin and MPA (Fig. 1C). The combination of these agents did not show cytotoxicity.
To compare the effect of a combination of rapamycin and MPA to that of either agent alone, the expression levels of TNF-α-induced pro-inflammatory genes such as IL-1β, IL-6, and IL-8 were measured using reverse transcription-PCR (RT-PCR) in HaCaT cells. HaCaT cells were pretreated with rapamycin, MPA, or a combination of rapamycin and MPA for 1 h, and then stimulated with TNF-α for 6 h. The expression of pro-inflammatory genes increased in TNF-α-stimulated HaCaT cells. The treatment of cells with rapamycin (100 nM) or MPA (100 nM) alone either slightly reduced or did not change the levels of IL-1β, IL-6, and IL-8 compared to the corresponding levels in the TNF-α-treated group. The treatment of cells with a combination of rapamycin (20 nM) and MPA (80 nM), or rapamycin (50 nM) and MPA (50 nM) only slightly reduced the level of pro-inflammatory gene expression, whereas rapamycin at 80 nM in combination with MPA at 20 nM strongly inhibited the levels of IL-1β, IL-6, and IL-8. An effect was not seen with any other single or combination treatment. Use of equal amounts of cDNA was confirmed on the basis of GAPDH expression (Fig. 2).
NF-κB is a transcription factor for genes encoding inflammatory cytokines, chemokines, and inflammation-related factors16. To verify the effects of combinations of rapamycin and MPA on TNF-α-induced NF-κB signaling cascades, the phosphorylation of IκBα and the translocation of NF-κB were measured by performing western blot. Cells were pretreated with rapamycin (100 nM), MPA (100 nM), or a combination of both drugs for 1 h, before being stimulated with TNF-α. The activation of NF-κB molecules was analyzed using either nuclear extracts or whole-cell lysates. As shown in Fig. 3A, treatment with rapamycin or MPA slightly inhibited the phosphorylation of IκBα, and similar results were obtained from the combination of rapamycin (20 nM) and MPA (80 nM) or rapamycin (50 nM) and MPA (50 nM). However, in the presence of rapamycin (80 nM) and MPA (20 nM), the inhibition of TNF-α-induced phosphorylation of IκBα was much higher than that with any other treatment. Moreover, a combination of rapamycin (80 nM) and MPA (20 nM) inhibited the TNF-α-induced nuclear localization of NF-κB p65 in HaCaT cells when compared with individual drug treatments and other combinations of the two drugs. These results suggest that the combination of rapamycin (80 nM) and MPA (20 nM) has an inhibitory effect on the signaling pathway that leads to the activation of NF-κB in TNF-α-treated HaCaT cells.
In order to assess the effects of a combination of these drugs on TNF-α-induced MAPK activation in HaCaT cells, we performed western blot analysis. We compared the inhibitory effects of a single drug or a combination of both drugs on the MAPK signaling pathway. As shown in Fig. 3B, in the presence of a combination of 80 nM rapamycin and 20 nM MPA, the inhibition of TNF-α-induced ERK activation was markedly higher than that in the presence of a combination of 50 nM rapamycin and 50 nM MPA. Single-drug treatment and the combination of 20 nM rapamycin and 80 nM MPA did not inhibit TNF-α-induced ERK activation. TNF-α-induced p38 activation decreased in the MPA-only treatment group and in the combination groups but not in the rapamycin-only treatment group.
To investigate the synergistic inhibitory effects of a combination of rapamycin and MPA on TNF-α-induced ICAM-1 and iNOS expression, RT-PCR and western blot analysis were performed. After TNF-α treatment in HaCaT cells, the HaCaT cells were treated with rapamycin and MPA alone, as well as with a combination of the two drugs. Following treatment, the levels of ICAM-1 and iNOS were measured by RT-PCR and western blot. TNF-α-induced ICAM-1 and iNOS expression in HaCaT cells was slightly suppressed by rapamycin and MPA. The co-treatment of rapamycin and MPA resulted in significantly greater inhibition compared to that with single-drug treatments. The greatest inhibitory effect was achieved with a combination of 80 nM rapamycin and 20 nM MPA (Fig. 4A).
In addition, we used pharmacological inhibitors of MAPK to investigate the functional relationship between MAPK activation and ICAM-1 expression. TNF-α-induced ICAM-1 expression was slightly suppressed in HaCaT cells with ERK, p38, and JNK inhibitors. These results suggest that the activation of ERK, p38, and JNK is involved in TNF-α-induced ICAM-1 expression, and the combination of rapamycin (80 nM) and MPA (20 nM) modulates signaling cascades that involve TNF-α-induced activation of ERK and p38 but not JNK (Fig. 4B).
Many immunosuppressive drugs are currently being used clinically, including cyclosporine, FK-506, rapamycin, and MPA25,26. Rapamycin has been used in the treatment of various pathophysiological conditions including nephrotoxicity, hypertension, ischemia, glutamate neurotoxicity, autoimmune diseases, and inflammatory diseases, by regulating the expression of nitric oxide and prostaglandin E227,28,29,30. MPA can induce the apoptosis of activated T-lymphocytes by depleting guanosine nucleotides, and suppress the expression and function of adhesion molecules required for the recruitment of lymphocytes and monocytes to sites of inflammation31,32.
Recently, synergistic or additive effects of combined treatments with rapamycin and MPA in various pathological conditions have been reported in several studies. Various studies suggest possible synergistic effects of a combination of rapamycin and imatinib on tuberous sclerosis complex neoplasia33, rapamycin and LY294002 in melanoma cells34, and rapamycin and cyclosporine A in an autoimmune disease model35.
In the present study, we found that the combination of rapamycin and MPA can synergistically inhibit pro-inflammatory reactions and molecular regulatory mechanisms in TNF-α-stimulated HaCaT cells.
There are many reports that an increase in the expression of nitric oxide and adhesion molecules such as ICAM-1 in keratinocytes plays an important role in diseases involving skin inflammation6,36,37. Our results indicate that a combination of rapamycin and MPA can inhibit TNF-α-induced ICAM-1 and iNOS expression in HaCaT cells. Interestingly, the combination of 80 nM rapamycin and 20 nM MPA resulted in significantly greater suppression than that achieved with single-agent treatments and combinations with different doses. We also observed a decrease in the expression of pro-inflammatory cytokines and chemokines in TNF-α-induced HaCaT cells.
This suggests that the anti-inflammatory effect of the combination of rapamycin and MPA is mediated by blocking the activation of NF-κB and the phosphorylation of ERK and p38 MAPK, which inhibits the expression of pro-inflammatory genes induced by TNF-α.
In conclusion, the findings of the present study demonstrate that a combination of rapamycin and MPA synergistically inhibits the expression of pro-inflammatory factors in TNF-α-induced HaCaT cells. These findings indicate that the inhibition of ICAM-1 expression might cause the blockade of IL-6 or IL-8 expression through inhibition of ERK, P38, and NF-κB signaling pathways by rapamycin and MPA. The use of lower concentrations of rapamycin and MPA are expected to reduce the occurrence of side effects and toxicity. The different anti-inflammatory mechanisms of rapamycin and MPA appear to result in a synergistic effect when the two drugs are used in combination. However, the interactions of these two immunosuppressive drugs are still being examined, and additional evaluations with other types of immune cells and in vivo trials are needed.
Figures and Tables
 | Fig. 1Effects of rapamycin, mycophenolic acid (MPA), or a combination of the two drugs on the viability of HaCaT cells. (A) Cytotoxic effects of rapamycin and MPA in HaCaT cells. The cells were treated with rapamycin and MPA in a dose-dependent manner for 24 h. (B) Confirmation of the cytotoxic effects of a combination of rapamycin and MPA in HaCaT cells. The cells were treated as indicated in (C). Cell viability was determined using MTT assay as described in the Materials and Methods. The measurements were performed in triplicate. TNF-α: tumor necrosis factor-α. |
 | Fig. 2Effects of a combination of rapamycin (RP) and mycophenolic acid (MPA) on tumor necrosis factor (TNF)-α-induced expression of pro-inflammatory genes in HaCaT cells. HaCaT cells were pre-incubated for 24 h and then stimulated with TNF-α (20 ng/ml) after a 1 h pretreatment with RP, MPA, or a combination of the two drugs for 6 h. Pro-inflammatory gene expression was analyzed by reverse transcription-polymerase chain reaction (RT-PCR) using specific primers. The experiment was repeated independently at least three times. IL: interleukin, GAPDH: glyceraldehyde 3-phosphate dehydrogenase. |
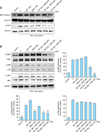 | Fig. 3Inhibitory effects of rapamycin (RP) and mycophenolic acid (MPA) on tumor necrosis factor (TNF)-α-induced activation of nuclear factor-kappa B (NF-κB), extracellular signal-related kinases (ERK), p38, and c-Jun N-terminal kinases (JNK) mitogen activated protein kinases (MAPKs) in HaCaT cells. HaCaT cells were pre-incubated for 24 h. The cells were then pre-treated with RP, MPA, or a combination of the two drugs for 1 h, and then stimulated by TNF-α (20 ng/ml) for 20 min. (A) The phosphorylation of IκB α and the translocation of NF-κB p65 in whole-cell protein lysates were determined by western blot analysis. Cell lysates were prepared and subjected to western blot with the indicated antibodies. (B) The phosphorylation of substrates in the MAPK family (ERK, p38, JNK) was detected by western blot analysis using specific antibodies. The densities of protein expression were expressed as percentages of protein density in the TNF-α-only group. *p<0.05 and **p<0.01 versus the TNF-α-treated group. The densitometric analysis of the western blot was standardized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. |
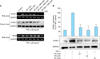 | Fig. 4Inhibitory effects of rapamycin (RP) and mycophenolic acid (MPA) on tumor necrosis factor (TNF)-α-induced intercellular adhesion molecule 1 (ICAM-1) and inducible nitric oxide synthase (iNOS) expression in HaCaT cells. (A) HaCaT cells were pre-incubated for 24 h, and then stimulated with TNF-α (20 ng/ml) in the presence of RP, MPA, or a combination of the two drugs for 3 h (for RNA) or 12 h (for protein). ICAM-1 and iNOS expression were determined after treatment with 100 nM RP, 100 nM MPA, and different combinations of the two drugs (20 nM RP and 80 nM MPA, 50 nM RP and 50 nM MPA, and 80 nM RP and 20 nM MPA) by reverse transcription-polymerase chain reaction (RT-PCR) and western blot. (B) HaCaT cells were pretreated with an extracellular signal-related kinases (ERK) inhibitor (PD98059), p38 inhibitor (SB203580), or c-Jun N-terminal kinases (JNK) inhibitor (SP600125) for 1 h, and then stimulated with TNF-α for 12 h. The band densities are expressed as percentages of the band density of the TNF-α-only group. **p<0.01 compared to treatment with TNF-α alone. The experiment was repeated independently at least three times. The densitometric analysis of the western blot was standardized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. |
ACKNOWLEDGMENT
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A092258).
References
1. Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993; 14:75–78.

2. Kupper TS. Mechanisms of cutaneous inflammation. Interactions between epidermal cytokines, adhesion molecules, and leukocytes. Arch Dermatol. 1989; 125:1406–1412.

3. Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991; 337:211–214.

4. Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007; 19:469–474.
5. Albanesi C, Cavani A, Girolomoni G. Interferon-gamma-stimulated human keratinocytes express the genes necessary for the production of peptide-loaded MHC class II molecules. J Invest Dermatol. 1998; 110:138–142.
6. Trefzer U, Brockhaus M, Loetscher H, Parlow F, Kapp A, Schöpf E, et al. 55-kd tumor necrosis factor receptor is expressed by human keratinocytes and plays a pivotal role in regulation of human keratinocyte ICAM-1 expression. J Invest Dermatol. 1991; 97:911–916.

7. Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004; 23:1902–1910.

8. Bachelez H. Immunopathogenesis of psoriasis: recent insights on the role of adaptive and innate immunity. J Autoimmun. 2005; 25:Suppl. 69–73.

9. Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010; 222:729–737.
10. Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004; 279:32633–32642.

12. Mercurio F, Young DB, Manning AM. Detection and purification of a multiprotein kinase complex from mammalian cells. IKK signalsome. Methods Mol Biol. 2000; 99:109–125.

13. Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001; 7:1291–1297.

14. Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996; 14:649–683.
15. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996; 87:13–20.
16. Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008; 128:2606–2614.

17. Mason J. Pharmacology of cyclosporine (sandimmune). VII. Pathophysiology and toxicology of cyclosporine in humans and animals. Pharmacol Rev. 1990; 41:423–434.
19. Huang S, Houghton PJ. Inhibitors of mammalian target of rapamycin as novel antitumor agents: from bench to clinic. Curr Opin Investig Drugs. 2002; 3:295–304.
20. Sabatini DM, Pierchala BA, Barrow RK, Schell MJ, Snyder SH. The rapamycin and FKBP12 target (RAFT) displays phosphatidylinositol 4-kinase activity. J Biol Chem. 1995; 270:20875–20878.

22. Epinette WW, Parker CM, Jones EL, Greist MC. Mycophenolic acid for psoriasis. A review of pharmacology, long-term efficacy, and safety. J Am Acad Dermatol. 1987; 17:962–971.
24. Allison AC, Eugui EM. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev. 1993; 136:5–28.

25. Langford CA, Klippel JH, Balow JE, James SP, Sneller MC. Use of cytotoxic agents and cyclosporine in the treatment of autoimmune disease. Part 2: Inflammatory bowel disease, systemic vasculitis, and therapeutic toxicity. Ann Intern Med. 1998; 129:49–58.

26. Taylor AC, Connell WR, Elliott R, d'Apice AJ. Oral cyclosporin in refractory inflammatory bowel disease. Aust N Z J Med. 1998; 28:179–183.

27. Bobadilla NA, Gamba G, Tapia E, García-Torres R, Bolio A, López-Zetina P, et al. Role of NO in cyclosporin nephrotoxicity: effects of chronic NO inhibition and NO synthases gene expression. Am J Physiol. 1998; 274:F791–F798.
28. Gerber DA, Bonham CA, Thomson AW. Immunosuppressive agents: recent developments in molecular action and clinical application. Transplant Proc. 1998; 30:1573–1579.

29. Hutcheson AE, Rao MR, Olinde KD, Markov AK. Myocardial toxicity of cyclosporin A: inhibition of calcium ATPase and nitric oxide synthase activities and attenuation by fructose-1,6-diphosphate in vitro. Res Commun Mol Pathol Pharmacol. 1995; 89:17–26.
30. Stein CM, Pincus T, Yocum D, Tugwell P, Wells G, Gluck O, et al. The Methotrexate-Cyclosporine Combination Study Group. Combination treatment of severe rheumatoid arthritis with cyclosporine and methotrexate for forty-eight weeks: an open-label extension study. Arthritis Rheum. 1997; 40:1843–1851.

31. Cohn RG, Mirkovich A, Dunlap B, Burton P, Chiu SH, Eugui E, et al. Mycophenolic acid increases apoptosis, lysosomes and lipid droplets in human lymphoid and monocytic cell lines. Transplantation. 1999; 68:411–418.

32. Blaheta RA, Leckel K, Wittig B, Zenker D, Oppermann E, Harder S, et al. Mycophenolate mofetil impairs transendothelial migration of allogeneic CD4 and CD8 T-cells. Transplant Proc. 1999; 31:1250–1252.

33. Govindarajan B, Willoughby L, Band H, Curatolo AS, Veledar E, Chen S, et al. Cooperative benefit for the combination of rapamycin and imatinib in tuberous sclerosis complex neoplasia. Vasc Cell. 2012; 4:11.

34. Werzowa J, Cejka D, Fuereder T, Dekrout B, Thallinger C, Pehamberger H, et al. Suppression of mTOR complex 2-dependent AKT phosphorylation in melanoma cells by combined treatment with rapamycin and LY294002. Br J Dermatol. 2009; 160:955–964.

35. Martin DF, DeBarge LR, Nussenblatt RB, Chan CC, Roberge FG. Synergistic effect of rapamycin and cyclosporin A in the treatment of experimental autoimmune uveoretinitis. J Immunol. 1995; 154:922–927.
36. Krutmann J, Czech W, Parlow F, Trefzer U, Kapp A, Schöpf E, et al. Ultraviolet radiation effects on human keratinocyte ICAM-1 expression: UV-induced inhibition of cytokine-induced ICAM-1 mRNA expression is transient, differentially restored for IFN gamma versus TNF alpha, and followed by ICAM-1 induction via a TNF alpha-like pathway. J Invest Dermatol. 1992; 98:923–928.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download