Abstract
Here, we report a case of Cowden syndrome with an unusual clinical course of late-onset oral papillomatosis and a novel germline PTEN mutation. Cowden syndrome is the most common phosphatase and tensin homolog hamartomatous tumor syndrome. It is characterized by multiple hamartomas in the gastrointestinal tract and mucocutaneous lesions such as trichilemmomas, oral papillomatosis, facial papules, and acral keratoses. Patients with Cowden syndrome have a higher risk of malignancies, especially breast, colon, and thyroid cancers. A 53-year-old female presented with cobblestone-like papillomatous papules on the lower gums that developed 1 year earlier. She had no other mucocutaneous lesions besides oral papillomatosis. Gastrointestinal endoscopy and colonoscopy revealed multiple hamartomas in the stomach and colon. The patient had a history of breast cancer and multinodular goiter diagnosed 4 and 5 years ago, respectively. She was diagnosed with Cowden syndrome and a novel PTEN mutation was confirmed by direct sequencing.
Cowden syndrome (CS) is a rare autosomal dominant multiple hamartoma syndrome but is the most common phosphatase and tensin homolog (PTEN) hamartomatous tumor syndromes, which includes Bannayan-Riley-Ruvalcaba syndrome, Proteus syndrome and Proteus-like syndrome1,2. Most CS cases are caused by mutations in the tumor suppressor gene PTEN and are characterized by multiple hamartomas in various organs and mucocutaneous alterations1. The risk of malignancy increases with cellular proliferation of all three embryonic germ cell lines3. Moreover, breast, colon and thyroid cancers are commonly associated with CS4.
Here, we report a case of CS presenting as a single mucocutaneous lesion of late-onset oral papillomatosis with a novel germline PTEN mutation.
A 53-year-old woman presented with multiple papules on the lower gums. This lesion developed one year earlier and spread over the gingiva. The patient had an unremarkable medical history until she underwent hysterectomy owing to uterine myoma 10 years earlier. Five years earlier, she was diagnosed with multinodular goiter. Then, 4 years ago, she was diagnosed with invasive apocrine-type ductal carcinoma of intermediate grade (score 7) in her left breast. Asymptomatic hamartomatous hyperplasia was subsequently found in her right cerebellum on brain magnetic resonance imaging (Fig. 1). She had never taken medications that could induce gum hypertrophy, such as cyclosporine, phenytoin, calcium channel blockers, etc. She had no family history of malignancy or genetic disorders.
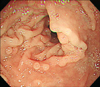
Physical examination showed multiple 2 to 7 mm skin-colored papules on the lower gums and adjacent gingiva, giving a cobblestone-like appearance (Fig. 2A). Histological examination of the papule on the lower gums revealed fibroepithelial hyperplasia with prominent inflammatory cell infiltration (Fig. 2B; H&E, ×40). There were no other remarkable dermatological findings on the face or body. The patient was referred to the Department of Gastroenterology to screen for the involvement of gastrointestinal systems. Upper gastrointestinal endoscopy and colonoscopy revealed multiple polyps in the gastric mucosa and rectum as well as around the ileocecal valve (Fig. 3).
A peripheral blood sample was obtained from the patient after she provided informed consent. Genetic analysis by direct sequencing of all nine exons of the PTEN gene and their flanking regions was performed as described previously5. Primers for the amplification of exon 9 were designed for this study. Sequence variation was described according to the recommendations of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/) using a cDNA reference sequence (NM.000314.4). Sequence analysis revealed a heterozygous mutation, c.987_990delTAAA, in exon 8 (Fig. 4). This four-nucleotide deletion (p.N329fs*14) in the C2 domain of PTEN protein is predicted to lead to a frame shift at amino acid position 329, resulting in premature translation termination at the 14th position from the altered site.
CS was first reported in 1963 by Lloyd and Dennis3 and is named after a patient who presented with multiple hamartomas, fibrocystic breast disease, central nervous system disease, and unusual skin lesions. It is the most common PTEN hamartomatous tumor syndrome caused by PTEN mutation1. PTEN, also known as MMAC1 or TEP16,7, is a tumor-suppressor gene that maps to 10q22~238. It encodes a dual-function phosphatase whose substrate is phosphatidylinositol, which is a phospholipid in the phosphatidylinositol 3-kinase pathway that shares homology with the adhesion molecules tensin and auxilin6,7,9. Using both protein and lipid substrates, it regulates cell cycling, cellular growth, proliferation, and angiogenesis10.
Although hamartomas can develop in almost all organs, they predominantly occur in the skin and gastrointestinal system in CS; The gastrointestinal system is involved in 70% to 85% of patients with CS. Polyps usually smaller than 5 mm occur predominantly in the esophagus, stomach, and colorectal structures but not the small intestine1. Mucocutaneous lesions are present in 90% to 100% of CS cases2. Oral papillomatosis is an important clinical manifestation for diagnosis and is usually located in the buccal and gingival mucosa where lesions coalesce and produce a cobblestone-like appearance. A histopathological feature of oral papillomatosis is inflammatory fibroepithelial hyperplasia10, which present in the case reported herein. Multiple hamartomas can also affect the bones, eyes, genitourinary tract and central nervous system1. Lhermitte-Duclos disease is a central nervous system disorder characterized by progressive hamartomatous enlargement of the cerebellum, as in the present case, which meets the pathognomonic criteria for the diagnosis of CS2,11.
The diagnostic criteria for CS were first proposed in 1996 by the International Cowden Syndrome Consortium and were revised in 2008 by the National Comprehensive Cancer Network12. They are based on the clinical manifestations and divided into pathognomonic, major and minor criteria. Pathognomonic lesions include adult-onset Lhermitte-Duclos disease, facial trichilemmomas, acral keratoses, and papillomatous lesions. Major criteria include breast cancer, thyroid cancer (i.e., papillary or follicular), macrocephaly, and endometrial cancer. Minor criteria include other structural thyroid lesions (e.g., adenoma and multinodular goiter), mental retardation, gastrointestinal hamartomas, fibrocystic disease of the breasts, lipomas, fibromas, genitourinary tumors, genitourinary structural malformations, and uterine fibroids. Diagnosis can be established when the patient meets any four of the criteria as suggested in the revised diagnostic criteria12.
The present patient presented with papillomatous papules in the oral cavity, breast cancer, multinodular goiter, hamartomatous hypertrophy of the cerebellum, uterine myoma and hamartomas in the gastrointestinal system; thus, the case met two pathognomonic criteria, one major criterion and three minor criteria. Therefore, the patient was diagnosed with CS according to the National Comprehensive Cancer Network criteria. Moreover, the molecular diagnosis of a germline PTEN mutation was established by direct PTEN sequencing, which revealed a C987-990-delTAAA frameshift mutation; this mutation has been reported as a somatic mutation in endometrial cancer and brain tumor tissues13,14,15. As a germline mutation, Marsh et al.16 report one case of this mutation in a Bannayan-Riley-Ruvalcaba/CS overlap family. However, the present case is thought to be the first case in a patient with pure CS.
Because mucocutaneous manifestations of CS are commonly observed among the general population2 and usually precede the development of neoplastic processes10,17, recognizing cases that involves only mucocutaneous lesions can be challenging. However, in the present case, oral papillomatosis developed after the involvement of other organs. This late-onset mucocutaneous lesion made it easy to recognize CS and enabled the immediate surveillance of other unaffected organs, such as endoscopic evaluation of the gastrointestinal system. In addition, unlike the usual presentation of CS, in which papillomatous or lichenoid papules mainly develop in the maxillofacial area, neck, backs of the hands, and forearms18, the present patient presented with a solitary mucocutaneous lesion as fibropapillomatosis of the oral mucosa. CS could not be considered in the differential diagnosis if the patient had no other manifestation of hamartomatous hyperplasia. Considering that oral papillomatosis can be the single mucocutaneous lesion in CS, as in this case, a multidisciplinary approach involving history taking and histopathological examination is necessary to differentiate CS from other conditions.
CS is rare hamartomatous syndrome in which malignancies can developed in multiple organs. Mucocutaneous lesions are found in the overwhelming majority of patients with CS and almost always appear before the initiation of neoplastic processes10. Therefore, recognizing benign manifestations is critical when diagnosing CS10. The present case exhibited an unusual clinical course in which oral papillomatosis occurred after the other fatal involvements of CS. This suggests the absence of a benign skin lesion cannot be used to exclude a diagnosis of CS. Furthermore, direct sequencing revealed a C987-990delTAAA frameshift mutation of the PTEN gene, which has never been reported in a patient with pure CS. Therefore, further studies are needed to clarify the association between the course of CS and the specific type of PTEN mutation.
Figures and Tables
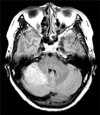 | Fig. 1Brain magnetic resonance imaging performed 4 years earlier showing a hyperintense lesion on the right cerebellum suggestive of hamartomatous hypertrophy. |
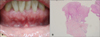 | Fig. 2(A) A 53-year-old woman who presented with multiple cobblestone-like papules on her lower gums. (B) Histopathological findings revealed prominent acanthosis and papillomatosis in the epidermis and proliferation of collagen fibers in the dermis (H&E, ×40). |
 | Fig. 4Electropherograms of the PTEN gene. Sequence analysis revealed a heterozygous TAAA deletion (underlined) between cDNA position 987 and 990. In the reverse strand (top), heterozygous peaks before the deletion site (987-990) represent a mixture of the deleted sequence (middle) and undeleted sequences (bottom). |
References
1. Shah KR, Boland CR, Patel M, Thrash B, Menter A. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013; 68:189.e1–189.e21.
3. Lloyd KM 2nd, Dennis M. Cowden's disease. A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963; 58:136–142.
4. McLean DI, Haynes HA. Cutaneous manifestation of intestinal malignant disease. In : Freedberg IM, Eisen AZ, Wolff K, Austen KF, Gold Smith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill;2003. p. 1789.
5. Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008; 118:3762–3774.

6. Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997; 15:356–362.

7. Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997; 57:2124–2129.
8. Tutluer S, Tanriover MD, Guven GS. Cowden syndrome: a major indication for extensive cancer surveillance. Med Oncol. 2012; 29:1365–1368.

9. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997; 275:1943–1947.

10. Mukamal LV, Ferreira AF, Jacques Cde M, Amorim CA, Pineiro-Maceira J, Ramos-e-Silva M. Cowden syndrome: review and report of a case of late diagnosis. Int J Dermatol. 2012; 51:1494–1499.

11. Eng C. PTEN hamartoma tumor syndrome (PHTS). In : Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle;2001. 11. 29. updated 2014 Jan 23. cited 2014 May 1. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1488/. .
12. Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns. 2009; 18:13–27.

13. Kanaya T, Kyo S, Sakaguchi J, Maida Y, Nakamura M, Takakura M, et al. Association of mismatch repair deficiency with PTEN frameshift mutations in endometrial cancers and the precursors in a Japanese population. Am J Clin Pathol. 2005; 124:89–96.

14. Schmidt EE, Ichimura K, Goike HM, Moshref A, Liu L, Collins VP. Mutational profile of the PTEN gene in primary human astrocytic tumors and cultivated xenografts. J Neuropathol Exp Neurol. 1999; 58:1170–1183.

15. Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997; 57:4736–4738.
16. Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, et al. PTEN mutation spectrum and genotypephenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999; 8:1461–1472.

17. Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005; 100:476–490.

18. Hildenbrand C, Burgdorf WH, Lautenschlager S. Cowden syndrome-diagnostic skin signs. Dermatology. 2001; 202:362–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download