Abstract
Background
There is no appropriate in vivo animal model that reflects the inflammatory response of human acne.
Objective
This study investigated the effect of Propionibacterium acnes on the development of inflammatory acne-like lesions in four mouse strains with different degrees of immune response for the development of an optimal mouse model of inflammatory acne.
Methods
Human P. acnes suspensions (108 and 109 colony forming unit [CFU]/µl) were injected into the backs of HR-1, BALB/c, vitamin D receptor-knockout (VDR k/o), and severe combined immunodeficiency disease mice. Inflammation levels were evaluated two weeks after injection of P. acnes suspensions. In addition, histopathological examination and immunohistochemical staining of the expressions of inflammatory biomarkers (i.e., CD4+/CD8+ T lymphocytes, neutrophils, myeloperoxidase, interleukin-1β, matrix metalloprotease (MMP)-2, MMP-3, MMP-9, toll-like receptor (TLR)-2, LL-37, and integrin α6) were performed on tissue specimens.
Results
The HR-1 mouse strain exhibited the most remarkable inflammatory reaction with epithelial proliferation and microcomedone-like cyst formation. HR-1 mice also demonstrated aberrant integrin expression in the epidermis around both inflamed lesions and newly formed microcomedones. These findings were more prominent in the group receiving 109 CFU/µl P. acnes than 108 CFU/µl. MMP-9 expression in HR-1 mice was also upregulated around the microcomedone-like cysts. Finally, expression levels of TLR-2 and LL-37 were higher in HR-1 and BALB/c mice than the VDR k/o and SCID mice strains.
Acne vulgaris is a multifactorial pleomorphic skin disease of the pilosebaceous follicles that is characterized by various non-inflamed and inflamed lesions1. The four major factors involved in its pathogenesis are increased sebum production, hypercornification of the pilosebaceous follicle, abnormality of the microbial flora (especially colonization of the ducts with Propionibacterium acnes), and the development of inflammation. In clinical practice, inflammation is the main source of discomfort and disfigurement in patients with acne2.
The initial lesions of acne are microcomedones. However, prior to the formation of a microcomedone, one of the characteristic findings leading to the development of acne lesions is hypercornification of the follicle wall. A previous study also suggests inflammatory events occur in advance of and act as possible causal factors in the hyperproliferative changes observed in acne lesions. P. acnes plays an important role in the induction of such inflammatory events3. In addition, P. acnes can induce abnormal proliferation and differentiation of keratinocytes1. Therefore, in early inflammatory acne lesions, P. acnes may act as a major factor contributing to microcomedone formation via the induction of inflammatory responses and hypercornification of the follicle wall.
The use of animal models for drug development has recently been increasing exponentially. Animal models have been employed to mimic both human skin conditions and diseases. Although there are various animal and human models of acnegenesis, such as the Mexican hairless dog, Rhino mouse and rabbit ear assay, no elucidative model that precisely reflects comedogenesis is available4. In addition, none of the aforementioned models approximate the inflammatory processes observed in human inflammatory acne lesions owing to immune deficits and lack of bacterial colonization.
Therefore, this study aimed to develop a simple mouse model system reflective of the processes of inflammation and comedogenesis by examining the effects of P. acnes in four mouse strains with differing degrees of immune responses.
To evaluate the degree of the inflammatory response required for acne vulgaris development, four mouse strains with varying immune responses were use. Six-week-old female Hos:HR-1 mice (HR-1; SLC Inc., Hamamatsu, Japan), six-week-old female BALB/c-nu Slc mice (BALB/c; SLC Inc., Hamamatsu, Japan), eight-week-old male or female C57BL/6J Vdr-/- mice (vitamin D receptor-knockout mice [VDR k/o]; CLEA Japan, Tokyo, Japan), and six-week-old female severe combined immunodeficiency mice (SCID; SLC Inc.) were kept under conventional laboratory conditions and tested after 1 week of acclimation. Two mice from each strain were used. The animal study protocol was approved by the ethics committee for animal studies at Kyungpook National University, Republic of Korea (permission number: KNU 2014-0135).
P. acnes strain (ATCC 1182) was isolated from the pustular lesions of Korean patients with moderate inflammatory acne. P. acnes from post-log phase cultures were grown on brain-heart infusion agar, harvested, heat inactivated at 95℃ for 5 minures, and lyophilized prior to injection. P. acnes suspensions were prepared at concentrations of 108 and 109 colony forming units (CFU)/µl. Using a 30-gauge needle, P. acnes suspensions were injected in 20-P. acnesl aliquots intradermally into both sides of the backs of the mice.
The degree of clinical inflammatory change was evaluated by digital photography at baseline and 2 weeks after P. acnes injection.
Two weeks after P. acnes injection, tissue samples from each mouse were obtained by excisional biopsy of the inflammatory nodule. Paraffin-embedded tissue sections 3 µm thick were processed routinely for light microscopy. Hematoxylin & eosin and immunohistochemical staining were performed using standard techniques. The primary antibodies were as follows: integrin α6 (1:150; Santa Cruz Biotechnology Inc., CA, USA), CD4+ T cells (1:300; Abcam, Cambridge, UK), CD8+ T cells (1:100; Abcam), neutrophil (1:80; Abcam), myeloperoxidase (MPO, 1:200; Abcam), interleukin (IL)-1β (1:150; Abcam), matrix metalloprotease (MMP-2, 1: 300; Abcam), MMP-3 (1:100; Abcam), MMP-9 (1:250; Abcam), toll-like receptor-2 (TLR-2, 1:500; Abcam), and LL-37 (1:300; Abcam). Histological changes were compared among the four mouse strains, specifically changes in inflammation, epidermal/follicular wall thickness, the formation of cystic structures containing keratinized plugs (i.e., microcomedone-like cystic structures) in the dermis, and inflammatory cells/markers. Tissue expression of each antibody was graded on a semiquantitative scale.
Two mice per strain were examined. In addition, one mouse of each strain was used as a control. Two weeks after injection with the lower concentration of P. acnes (108 CFU/µl), the most severely inflamed nodule developed in the HR-1 mice (Table 1, Fig. 1). Similar results were observed following injection with the higher-concentration P. acnes suspension (109 CFU/µl). Inflammatory responses evaluated on the basis of nodule size were dependent on the concentration of P. acnes injected.
Inflammatory responses were observed histologically in all mouse strains. Interestingly, epidermal hyperplasia and thickening were significantly greater in HR-1 mice than BALB/c mice at both P. acnes concentrations (Fig. 2). In addition, several microcomedone-like cysts were observed around the focus of inflammation in HR-1 mice (Fig. 3). However, despite their severe inflammatory response, these changes were not observed in BALB/c mice. Neither epidermal changes nor the formation of microcomedone-like cysts was observed in the VDR k/o or SCID mice (Table 1). It should be noted that microcomedone-like cysts can develop in VDR k/o mice due to hypocalcemia but not from P. acnes exposure.
Nodular CD4+/CD8+ T cell infiltration was more prominent in HR-1 and BALB/c mice than the VDR k/o and SCID mice. In addition, neutrophils were significantly elevated at the focus of inflammation induced by P. acnes in all four mouse strains. MPO expression was also detected throughout the inflammatory focus in all four strains (Table 1).
In order to identify an appropriate mouse strain for the development of a simple inflammatory acne mouse model, we injected P. acnes into the dorsal skin of four strains of mice with varying levels of immune response. Injected P. acnes may induce the granulomatous type of acne inflammation that follows follicular rupture. In this study, the acute inflammatory response induced by P. acnes generated epidermal hyperplasia followed by the formation of secondary microcomedones. Interestingly, the severity of epidermal hyperplasia, as demonstrated by the presence of integrin α6, was proportional to the inflammatory response induced by P. acnes. In addition, many microcomedone-like cysts formed in the case of severe epidermal thickening. Such reaction processes were most remarkable in and appeared to be specific to HR-1 mice. Thus, a unique genetic abnormality may exist in the HR-1 hairless mouse strain that is required for the manifestation of the observed skin lesions. HR-1 mice lack the repressor protein HR, which leads to altered transcription of gene products that function in keratinocyte differentiation5. Mutations that affect keratinocyte gene expression may alter thymus development and cell-mediated immunity, as is dramatically illustrated by the nude phenotype due to homozygous disruption of Foxn1. Therefore, mutations in the HR gene have the potential to seriously impact immunologic function, which underscores the purpose of this study to evaluate the effects of P. acnes on HR-1 mice6.
BALB/c mice are among the most widely used inbred strains in animal experimentation; they are ideal for general multipurpose models, hybridoma development, monoclonal antibody production, and infectious disease research. Moreover, they are susceptible to a variety of bacterial, viral, and fungal infections; this increased susceptibility appears to be due to the lack of mediated immunity in these athymic mice7. Meanwhile, VDR k/o mice are characterized by severe hypocalcemia and exhibit important defects in macrophage functioning and cellular immunity in vivo8. In addition, they exhibit early alopecia, thickened skin, enlarged sebaceous glands, and epidermal cyst development9. SCID mice exhibit severe combined immunodeficiency that affects both B and T lymphocytes. Therefore, SCID mice have the lowest levels of immune response among the four mouse stains tested10.
Although the four strains utilized in this study have different immune response deficiency levels and cutaneous characteristics, the inflammatory responses and microcomedone-like cyst formation after P. acnes injection were most prominent in HR-1 mice. This suggests that there is a direct and sequential correlation among the following three factors: acute inflammation, epidermal changes, and microcomedone-like cyst formation. Previous studies indicate inflammation has always been considered a secondary event preceded by hyper-cornification of the follicular duct via hyper-proliferation11,12. However, Jeremy et al.3 show that inflammatory events occur in advance of the hyper-proliferative changes observed in acne lesions and can thus act as possible causal factors thereof, as opposed to being secondary consequential events. The results of the present study corroborate these findings.
The establishment of an in vivo acne mouse model is essential for understanding acnegenesis and screening anti-acne agents for acne prevention and control. Most animals do not produce sufficient triglycerides to harbor P. acnes13. Accordingly, dogs have no detectable triglycerides in their sebaceous glands, while mice, rabbits, and hamsters only have low triglyceride concentrations13. Therefore, the creation of an animal model harboring P. acnes requires external injection of this anaerobe. Various existing animal models for studying acne use this strategy; among them, rabbit ears and Rhino mice are commonly used to determine the compound comedogenicity of acne lesions14,15. However, the rabbit ear model does not include bacterial colonization and inflammation4,15. The use of rabbits may also be inconvenient for vast drug screening and vaccination protocols. On the other hand, mutant Rhino mice cannot elicit antibodies against thymus-dependent antigens16. Furthermore, in previous mouse studies, P. acnes was usually injected into the ears. However, because of the frailty of the mouse ear, the appearance of secondary changes due to inflammatory reaction is limited. Therefore, we injected P. acnes into the mouse dorsum and were readily able to observe the initial processes of inflammatory acne. On the basis of the results of this study, we propose HR-1 mice are appropriate for the development of an inflammatory animal model of acne.
Enzymes in the MMP family play a significant role in many biological activities, including multiple aspects of the immune response17. In general, MMPs are endopeptidases responsible for degrading components of the extracellular matrix, such as collagen and proteoglycans. Moreover, as potent chemokine antagonists, they play an important role in leukocyte migration and tissue remodeling. MMP-2 and MMP-9 are of particular interest in inflammatory models18. In the present study, MMP-9 expression was significantly elevated around microcomedone-like cysts in HR-1 mice compared to MMP-2 and MMP-3 levels. These findings suggest MMP-9 may play a critical role in the processes of dermal remodeling and cyst formation.
P. acnes is a key etiological factor in the development of inflammatory acne. Several additional factors such as lipids may also act as important etiologic agents; we are currently investigating them through the co-delivery of human sebocytes or culture supernatants along with P. acnes suspensions and measuring any improvement in their ability to produce acute inflammation and microcomedone cysts in HR-1 mice.
In conclusion, intradermal injection of P. acnes induces a focus of acute inflammation, hyperplastic changes in the epidermis, and the formation of microcomedone-like cysts in mice. Furthermore, various mouse strains differ with respect to their ability to exhibit changes that reflect the early inflammatory response of human acne. Finally, HR-1 mice are suitable for the development of a mouse model of inflammatory acne.
Figures and Tables
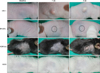 | Fig. 1Two weeks after injection with 108 colony forming unit/µl Propionibacterium acnes suspension, clinical inflammation was significantly more prominent of HR-1 mice than the three other strains. The extent of inflammation tended to be proportional to the concentration of injected P. acnes. VDR k/o: vitamin D receptor-knockout mice, SCID: severe combined immunodeficiency mice. |
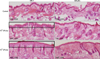 | Fig. 2Injected HR-1 mice exhibited significantly greater epidermal thickening than BALB/c mice. The degree of epidermal thickening was also dependent on the concentration of injected Propionibacterium acnes. CFU: colony forming unit. |
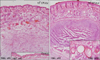 | Fig. 3Injected HR-1 mice exhibited several microcomedone-like cysts around the inflammatory focus induced by Propionibacterium acnes injection. CFU: colony forming unit. |
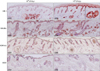 | Fig. 4Integrin α6 expression was significantly higher in the lower epidermis of injected HR-1 mice (particularly at 109 CFU/µl) than the other three mouse strains (staining method: H&E). CFU: colony forming unit, VDR k/o: vitamin D receptor-knockout mice, SCID: severe combined immunodeficiency mice. |
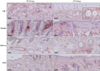 | Fig. 5Injected HR-1 and BALB/c mice exhibited higher toll-like receptor-2 expression in the epidermis than VDR k/o and SCID mice (staining method: H&E). CFU: colony forming unit, VDR k/o: vitamin D receptor-knockout mice, SCID: severe combined immunodeficiency mice. |
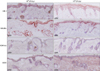 | Fig. 6Matrix metalloprotease-9 expression was significantly higher in the dermis of injected HR-1 mice than the other three strains; expression was more prominent around microcomedone-like cysts (staining method: H&E). CFU: colony forming unit, VDR k/o: vitamin D receptor-knockout mice, SCID: severe combined immunodeficiency mice. |
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2007017).
References
1. Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013; 27:1–10.
2. De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM. Intradermal injection of Propionibacterium acnes: a model of inflammation relevant to acne. J Invest Dermatol. 1984; 83:394–398.

3. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003; 121:20–27.

5. Liu Y, Sundberg JP, Das S, Carpenter D, Cain KT, Michaud EJ, et al. Molecular basis for hair loss in mice carrying a novel nonsense mutation (Hrrh-R) in the hairless gene (Hr). Vet Pathol. 2010; 47:167–176.

6. Schaffer BS, Grayson MH, Wortham JM, Kubicek CB, McCleish AT, Prajapati SI, et al. Immune competency of a hairless mouse strain for improved preclinical studies in genetically engineered mice. Mol Cancer Ther. 2010; 9:2354–2364.

7. Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980; 27:461–467.

8. Mathieu C, Van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, et al. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res. 2001; 16:2057–2065.

9. Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykkö I, et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009; 115:91–97.

10. Milner JD, Fasth A, Etzioni A. Autoimmunity in severe combined immunodeficiency (SCID): lessons from patients and experimental models. J Clin Immunol. 2008; 28:Suppl 1. S29–S33.

12. Cunliffe WJ, Holland DB, Clark SM, Stables GI. Comedogenesis: some new aetiological, clinical and therapeutic strategies. Br J Dermatol. 2000; 142:1084–1091.

13. Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981; 41:1269–1270.

14. Nakano K, Kiyokane K, Benvenuto-Andrade C, González S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol Physiol. 2007; 20:29–36.

15. Nakatsuji T, Shi Y, Zhu W, Huang CP, Chen YR, Lee DY, et al. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008; 8:3406–3415.

16. Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980; 40:27–32.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download