Abstract
Background
Para-phenylenediamine (PPD) is the primary patch test screening agent for hair dye contact allergy (HDCA). However, no recent studies have been published that describe the results of reactions to patch tests using PPD and hair dyes in Korea.
Objective
To analyze the results of the patch tests to PPD using the thin-layer rapid use epicutaneous (TRUE) Test system in patients with HDCA and to investigate patients' awareness that hair dyes contains allergens, which cause the development of HDCA.
Methods
Eighty-four patients with suspected HDCA (32 men and 52 women) underwent patch testing using the TRUE Test system and their own hair dyes. The patients' demographic data, behavior associated with hair dyeing, and clinical manifestations of HDCA were examined retrospectively.
Results
Positive patch-test reactions to hair dyes occurred in 53.6% of patients who used hair dyes, and they were diagnosed with HDCA. Although there was a significant correlation between HDCA and PPD sensitization (p=0.001), only 40% of the patients with HDCA showed positive reactions to PPD. Of the 45 patients diagnosed with HDCA, only 7 (15.6%) were aware that their hair dyes contained allergens that caused HDCA.
When allergic contact dermatitis (ACD) is caused by hair dye, it is known as a hair dye contact allergy (HDCA), and it is usually observed among people who dye their hair and among hairdressers. Para-phenylenediamine (PPD) is a chemical within many hair dyes that is a major cause of HDCA1,2. Although PPD was the most common cause of ACD in the 1970s, it received less attention than ammoniated mercury and nickel sulfate during the 1980s and 1990s, respectively, because of changes in the natural environment3. However, since the beginning of the 2000s, PPD has been receiving attention again as an allergen because of the heightened demand for hair dyeing, particularly among middle-aged individuals4.
PPD is highly irritable and toxic, and the American Contact Dermatitis Society considered it the allergen of the year in 2006. PPD was banned in Sweden and France, but its use was resumed later in the European Union1. Standard manufacturing practices for quasi-drugs established by the Korea Food and Drug Administration state that the permissible level of PPD is 3%; consequently, most hair dyes on the Korean market contain PPD at a level of ≤3%. Patients with ACD caused by toluene-2, 5-diamine or aminophenol, which can cross-react with PPD, have been reported, but the most effective marker of HDCA is PPD5,6,7. Additional studies on other agents that cause HDCA may be required in the future.
The thin-layer rapid use epicutaneous (TRUE) Test (Smart-Practice, Phoenix, AZ, USA) is a patch test that was introduced to Korea in 2005. Subsequently, it replaced the Korean standard series 25, because it has a quantitative antigen, shows a high level of reproducibility, and is easy to use in the diagnosis of ACD8. The TRUE Test contains 24 antigens that are considered the causative agents in approximately 80% of all ACD cases. Antigen number 20 in the TRUE Test system is comprised of 90 µg/cm2 of PPD. No studies in Korea have described the simultaneous sensitivities to hair dyes used by individuals or the PPD based on the results from patch tests using the TRUE Test system. In this study, the patch tests were conducted with hair dyes used by the patients to determine the PPD contribution to HDCA, and the PPD-positive rate determined by the TRUE Test was investigated in those patients who showed positive reactions to their own hair dyes. Lastly, we also investigated the patients' awareness about hair dyes as agents that cause HDCA.
Between July 2011 and June 2013, this retrospective study investigated 113 patients who were suspected to have HDCA and encouraged to undergo patch testing using their hair dyes and the TRUE Test system at the Dermatology Department of Kangdong Sacred Heart Hospital at Hallym University in Korea. Patients who were suspected to have HDCA underwent patch tests if they repeatedly experienced eczema with pruritus after dyeing their hair, particularly on the scalp and face, and if they had a history of allergic reactions to the hair dye. To eliminate confounding factors that could affect the sensitivity of the patch tests, we excluded 16 patients who had used immunosuppressive agents, including corticosteroids, within 2 weeks of the patch test; 8 patients with a history of atopic dermatitis; and 5 patients with a history of psoriasis. Therefore, 84 subjects (32 [38.1%] men and 52 [61.9%] women) were selected to participate in the study. The mean age of the study population was 60.0 years (range, 25~85 years).
All patients were patch tested using the standard technique with Finn Chambers (SmartPractice, Phoenix, AZ, USA), which were mounted on Scanpor tape (Actavis Norway AS, Norgesplaster, Norway). In this study, each subject's own hair dye products were patch tested as is, and we did not use any petrolatum or saline as a vehicle. The hair dyes were composed of a hair coloring solution, which is the first component, and an oxidizing solution, which is the second component. Thus, these two materials were placed separately in the Finn Chamber using cotton swabs or spatulas without further dilution. The Finn Chamber was secured to the patients' back, abdomen, or arm using the Scanpor tape and the TRUE Test. The position of the devices used for patch testing was determined by the presence of inflammatory skin lesions that could affect the results. Patch sites were marked with gentian violet for more precise visual grading and to ensure that the patches adhered to the identical sites each day for the test duration. Patients were instructed to keep the patch sites clean and dry.
The patch-test results were read twice. An antigen was attached to the subject's skin, which was sealed and then removed after 48 h. The primary reading was performed 30 min after the removal of the antigen, and the secondary reading was performed 48 h after the first reading. Irritant reactions due to hyperirritable skin were excluded by a trained dermatologist, and the test was repeated in 1 month when the patient's skin became less irritable. Based on the International Contact Dermatitis Research Group's criteria, the patch-test results were categorized as negative (-), doubtful (?), weakly positive (+), strongly positive (+ +), or extremely positive (+++). A positive reaction was defined as at least one reading (either the primary or secondary) that was weakly positive (+) or higher. When a positive reaction to the hair dye was observed in a patch test, HDCA was definitively diagnosed (Fig. 1).
Information about the clinical manifestations of HDCA was retrospectively collected from the subjects' electronic medical records and their photographic assessments. The electronic medical records included the subjects' age, sex, the numbers of years they had been dyeing their hair, the intervals between hair dyeing and the onset of dermatitis, the frequency of hair dyeing, where the hair dyeing was performed, and the histories of dermatitis once the investigations about hair dyeing had been initiated. Using clinical images, the distribution of the inflammatory lesions and their associated symptoms were investigated. When the information was incomplete, telephone calls and e-mail interviews were performed to gather missing information.
In this study, the positive reaction rates to the subjects' hair dyes and the positive reaction rates to the PPD TRUE Test were compared to determine whether the commonly used TRUE Test effectively diagnoses HDCA. The subjects were initially divided into two groups depending on whether they showed positive or negative reactions to the hair dyes in the patch tests. The patch test results to PPD from each group were analyzed using a chi-square test. The patients' demographic information, behaviors associated with hair dyeing, and histories were also analyzed. The clinical characteristics of HDCA, including its skin distribution and symptoms, were analyzed using multi-response frequency analysis. Associations between the ratio of the subjects who acknowledged that hair dyes contain allergens and the clinical symptoms were also analyzed. All statistical analyses were performed using PASW Statistics 18.0 (IBM Co., Armonk, NY, USA). A p-value ≤ 0.05 was considered statistically significant.
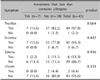
Of the 84 subjects, 45 (53.6%) had positive patch-test reactions to the hair dyes that they regularly use, and they were diagnosed with HDCA. Of those 45 individuals, 18 (40.0%) had positive patch-test reactions to PPD, and 27 (60.0%) had negative patch-test reactions to PPD. Of the 84 study participants, 21 (25.0%) had positive reactions to PPD. A statistically significant correlation was observed (p=0.001) between the ratio of the subjects who had positive reactions to the hair dyes that they regularly use and the ratio of the subjects who had positive reactions to PPD (Table 1).
The 45 subjects who were diagnosed with HDCA were divided into two groups: those who reacted positively and those who reacted negatively to PPD. The differences between the groups in relation to age, sex, the number of years spent dyeing hair, the interval between hair dyeing and the onset of dermatitis, the frequency of hair dyeing, the location at which the hair dyeing was performed, and a history of dermatitis following hair dyeing were investigated; however, no statistically significant results were found (p>0.05). Among the 45 subjects who were diagnosed with HDCA, 15 (33.3%) had dyed their hair for 1~5 years, 14 (31.1%) had dyed their hair for >10 years, and 8 (17.8%) had dyed their hair for 5~10 years. Of those 45 subjects, only 1 (2.2%) was diagnosed with HDCA after using hair dye for the first time. In relation to the interval between hair dyeing and the onset of dermatitis, 16 (35.6%) subjects had dermatitis after an interval of 1 week, 14 (31.1%) had dermatitis within 3 days, and 7 (15.6%) had dermatitis within 1 day of dyeing their hair. In relation to the frequency of hair dyeing, 17 (37.8%) subjects dyed their hair >10 times per year, 14 (31.1%) dyed their hair >6 times per year, and 10 (22.2%) dyed their hair >3 times per year. In terms of the location where the hair dyeing was performed, 21 (46.7%) subjects dyed their hair at home while 21 (46.7%) had their hair dyed at a salon. Patients with no history of dermatitis following hair dyeing were more common (25 subjects, 55.6%) than those with a history of dermatitis following hair dyeing (20 subjects, 44.4%) (Table 2).
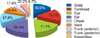
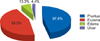
Among the 45 subjects diagnosed with HDCA, the forehead was the most common location for an allergic reaction (33 subjects, 73.3%), followed by the eyes (32 subjects, 71.1%) and the ears (31 subjects, 68.9%) (Fig. 2). Although the scalp is the area in which hair dyes have the greatest direct contact, this area showed a lower frequency as a location for allergic reactions (27 subjects, 60.0%) compared to the aforementioned areas (Table 3). The most common clinical symptom associated with HDCA was pruritus (44 subjects, 97.8%) followed by eczema (42 subjects, 93.3%) (Fig. 3). Of the 45 subjects who were diagnosed with HDCA, only 7 (15.6%) were aware that hair dyes are allergenic. Associations between the patients' awareness that hair dyes contain allergens and the presence of pruritus, eczema, ulcers, and edema were analyzed; however, no statistically significant results were obtained (Table 4). This suggests that regardless of the clinical symptoms, most of the patients with HDCA were not aware that their hair dyes are allergenic.
Hair is not just a skin appendage; it is used to express people's individuality, wellbeing, and attributes. Consequently, hair dyeing is widely accepted among younger people as a way to express youthfulness and color preferences, and among older people as a way to darken their graying hair9. The size of the Korean market for hair dye is expected to grow further with increases in women's social activities and the mean life span.
Modern hair dyeing began in 1893 when Hoffmann of Germany synthesized PPD and dyed hair at a low temperature, and this helped formed the basis of the oxidative hair dyes that are widely used today10. Oxidative hair dyes are composed of a hair coloring solution, which is the first component, and an oxidizing solution, which is the second component. Immediately before dyeing the hair, the two solutions are mixed, and then they are applied to the area to be dyed and are left on for some time. During this time, bleaching caused by the hydrogen peroxide in the second component and polymerization of the hair dye occur simultaneously to produce a new color. As the hair is dyed, the compounds in the hair dye are absorbed by the skin, which induces changes in the stratum corneum, and this may be accompanied by direct damage to the hair structure, leading to the development of ACD11. In Denmark, Søsted et al.12 showed that 5.3% of people who dye their hair experience adverse allergic skin reactions. In Korea, ACD is not as widely acknowledged as skin damage that is caused by hair dyes, and most patients do not understand that ACD is a consequence of skin damage caused by hair dyes, despite them being classified as medicinal products.
The most frequently suspected allergen that causes HDCA is PPD. The PPD sensitization rate within the general population is 1%~6%, and among patients who are highly likely to have HDCA, the PPD sensitization rate is reportedly 38%~97%13. Accordingly, PPD can be used as an effective marker of HDCA. PPD is included in the TRUE Test as antigen number 20, and positive reactions to PPD have gradually increased over time. Thyssen and White1 reported that the median prevalence of PPD sensitization in Asia was 4.3%, and it is consistently increasing. Patel et al.'s4 study showed that the rate of PPD sensitization rose sharply from 3.8% in 1989 to 7.1% in 2004 in the United Kingdom. A retrospective study conducted by Handa et al.14, which targeted 116 patients in India between 2009 and 2010, reported positive reactions to PPD in 19 (16.4%) patients, and that was an 11.7% increase from that found in a previous study that was conducted in the same country between 1999 and 2000. In a Korean study conducted by Lee et al.3, the positive reaction rate to PPD was reported as 5.7% between 2000 and 2006, and Hong et al.8 reported a positive reaction rate to PPD of 8.4% between 2009 and 2010, which was higher than the rate reported in a previous study3. The increase in PPD sensitization relates to the increase in the demand for hair dyes. Furthermore, PPD is not only present in hair dyes, but it is also present in henna tattoos, which are popular among younger people and are considered to have contributed to the increase in PPD sensitization15,16,17,18.
Of the 84 subjects in the current study who underwent patch tests because they were suspected to have HDCA, 45 (53.6%) had positive reactions to the hair dyes that they regularly use, and they were definitively diagnosed with HDCA. Of the 84 study subjects, 21 (25.0%) had a positive reaction to PPD in the TRUE Test, and of the 45 subjects who were diagnosed with HDCA, 18 (40.0%) had a positive reaction to PPD in the TRUE Test. Therefore, PPD was confirmed as an effective marker of HDCA in the current study on the basis of the statistically significant correlation between the positive reaction rates to the hair dyes that the subjects regularly use and the positive reaction rates to PPD, and this finding concurred with those from previous studies. However, since only 40% of the subjects with HDCA had positive reactions to PPD, additional studies to determine and validate other markers of HDCA in patients who show a negative reaction to PPD may be required. For example, additional patch tests with toluene-2,5-diamine, p-aminophenol, m-aminophenol, and o-aminophenol, which with the exception of PPD are the most frequently documented ingredients in hair dyes, may help to analyze the contribution of each of these ingredients to HDCA6,19,20.
Among the 45 subjects who were diagnosed with HDCA in this study, hair dyeing had most commonly been performed for 1~5 years (15 subjects, 33.3%). In many patients, the onset of dermatitis occurred within 1 week of the hair being dyed (16 subjects, 35.6%). Hair was commonly dyed >10 times per year (17 subjects, 37.8%). However, these behaviors associated with hair dyeing did not correlate with the positive patch test reactions to PPD. HDCA was most commonly observed on the subjects' foreheads (33 subjects, 73.3%), and pruritus was the most common clinical symptom (44 subjects, 97.8%). When comparing our study's findings to those reported by Søsted et al.7, the frequencies of edema and ulceration in our study were significantly lower than that of pruritus. In the present study, no severe acute allergic reactions such as conjunctivitis, dizziness, swollen lymph nodes, breathing difficulties, or headaches occurred, which Søsted et al.7 previously reported. Moreover, higher levels of allergic reactions were observed near the forehead, eyes, and ears than on the scalp, which is the area that has the greatest direct contact with hair dyes. Furthermore, the relatively mild clinical symptoms and the mismatch between the distribution of the skin lesions and the application area for the hair dye may mean that patients with HDCA fail to suspect that hair dyes contain allergens.
The most important point to note from this study is that of the 45 subjects who were diagnosed with HDCA, only 7 (15.6%) identified hair dyes as containing allergens. Indeed, only a few subjects who had allergic reactions to the hair dyes accepted that the hair dye they were using contained an allergen. However, people who have had HDCA at least once may develop considerably more severe allergic reactions if they are exposed to the same allergen again; thus, it is very important to accurately diagnose the condition and its underlying causes. To minimize the risk associated with allergic reactions, patients must understand the pathogenesis and clinical course of HDCA and also avoid hair dyeing. If this is unavoidable, patch tests are recommended before hair dyeing is attempted. As shown in this study, hair has often been dyed for years with few severe adverse reactions; therefore, most patients do not suspect that their hair dyes contain major allergens that cause the development of ACD. Besides, patients often reject the concept that hair dyes contain allergens even when dermatologists advise them of this possibility. As stated previously, hair dyes are barely acknowledged as the primary causative agents for the HDCA that develops in areas other than the scalp. Hence, patients suspected to have HDCA must be informed about the importance of undergoing patch tests. However, while the patch test is regarded as a gold standard method for diagnosing ACD, its utility is a topic of ongoing debate21. Despite efforts to standardize the allergens, vehicles, and concentrations to enhance the reproducibility of the patch tests, it is often difficult to identify the causative allergens because of the test techniques, reading or exposure times, and cross-reactions22,23,24,25. Therefore, patch tests should be conducted and interpreted by experienced dermatologists. Furthermore, since PPD can cross-react with its own derivatives and other components in hair dyes and induce irritant reactions as well as typical allergic reactions, greater attention should be paid to the interpretation of patch-test results.
The significance of this study lies in its focus on PPD sensitization, the behaviors associated with hair dyeing, and the clinical characteristics of Korean HDCA cases that have not been actively investigated before, despite their frequent occurrence. Nevertheless, since this study was conducted at a university hospital and it only targeted urban patients, it was limited because it did not emulate PPD sensitization across the entire Korean population. Further large-scale multicenter studies that investigate PPD sensitization categorized according to regional and demographic characteristics may help explain the prevalence and clinical features of HDCA in Korea. In addition, feasibility studies that investigate the commercialization of patch tests, which include the components of hair dyes other than PPD, may be required to help explain the mechanisms underlying HDCA, its diagnosis, and treatment.
Figures and Tables
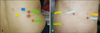 | Fig. 1Patch-test readings after 96 hours. (A) Positive reactions to two different types of hair dye components (para-phenylenediamine [PPD] and urushiol) are shown in patient 1. (B) Positive reactions to three different types of hair dye components and PPD are shown in patient 2. Doubtful reactions to nickel and chrome are also shown. |
Table 1
Associations between the positive patch-test reactions to the patients' hair dyes and the reactions to para-phenylenediamine (PPD)

References
1. Thyssen JP, White JM. European Society of Contact Dermatitis. Epidemiological data on consumer allergy to p-phenylenediamine. Contact Dermatitis. 2008; 59:327–343.
2. Malvestio A, Bovenzi M, Hoteit M, Belloni Fortina A, Peserico A, Corradin MT, et al. p-Phenylenediamine sensitization and occupation. Contact Dermatitis. 2011; 64:37–42.

3. Lee GY, Kim JK, Kim KJ. A study of the results of patch test in patients with contact dermatitis. Korean J Dermatol. 2007; 45:908–914.
4. Patel S, Basketter DA, Jefferies D, White IR, Rycroft RJ, McFadden JP, et al. Patch test frequency to p-phenylenediamine: follow up over the last 6 years. Contact Dermatitis. 2007; 56:35–37.

5. Orton D, Basketter D. Hair dye sensitivity testing: a critical commentary. Contact Dermatitis. 2012; 66:312–316.

6. Søsted H, Rastogi SC, Andersen KE, Johansen JD, Menné T. Hair dye contact allergy: quantitative exposure assessment of selected products and clinical cases. Contact Dermatitis. 2004; 50:344–348.

7. Søsted H, Agner T, Andersen KE, Menné T. 55 cases of allergic reactions to hair dye: a descriptive, consumer complaint-based study. Contact Dermatitis. 2002; 47:299–303.

8. Hong YJ, Choi HY, Kim KJ, Lee GY, Kim DW, Kim SJ, et al. TRUE test in patients with contact dermatitis: a multicenter study. Korean J Dermatol. 2011; 49:661–669.
9. Kim JE, Jung HD, Kang H. A survey of the awareness, knowledge and behavior of hair dye use in a Korean population with gray hair. Ann Dermatol. 2012; 24:274–279.

10. Ha BJ, Jeon DW, Kim KS. A study on the physico-chemical property evaluation of oxidative permanet hair color products containing p-phenylene diamine. J Fash Bus. 2005; 9:136–144.
11. Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009; 61:269–275.

12. Søsted H, Hesse U, Menné T, Andersen KE, Johansen JD. Contact dermatitis to hair dyes in a Danish adult population: an interview-based study. Br J Dermatol. 2005; 153:132–135.

13. Pot LM, Scheitza SM, Coenraads PJ, Blömeke B. Penetration and haptenation of p-phenylenediamine. Contact Dermatitis. 2013; 68:193–207.
14. Handa S, De D, Mahajan R. Epidemiological trends in contact dermatitis to hair dye: Comparing para-phenylenediamine positivity after a decade long interval. Indian J Dermatol Venereol Leprol. 2011; 77:511–512.

15. Sosted H, Johansen JD, Andersen KE, Menné T. Severe allergic hair dye reactions in 8 children. Contact Dermatitis. 2006; 54:87–91.

16. Almeida PJ, Borrego L, Limiñana JM. Age-related sensitization to p-phenylenediamine. Contact Dermatitis. 20111; 64:172–174.

17. Chung WH, Wang CM, Hong HS. Allergic contact dermatitis to temporary tattoos with positive para-phenylenediamine reactions: report of four cases. Int J Dermatol. 2001; 40:754–756.

18. Pegas JR, Criado PR, Criado RF, Vasconcellos C, Pires MC. Allergic contact dermatitis to temporary tattoo by p-phenylenediamine. J Investig Allergol Clin Immunol. 2002; 12:62–64.
19. Søsted H, Basketter DA, Estrada E, Johansen JD, Patlewicz GY. Ranking of hair dye substances according to predicted sensitization potency: quantitative structure-activity relationships. Contact Dermatitis. 2004; 51:241–254.

20. Søsted H, Rustemeyer T, Gonçalo M, Bruze M, Goossens A, Giménez-Arnau AM, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013; 69:32–39.

21. Wolf R, Orion E, Ruocco V, Baroni A, Ruocco E. Patch testing: facts and controversies. Clin Dermatol. 2013; 31:479–486.

22. Geier J, Ballmer-Weber BK, Dickel H, Frosch PJ, Bircher A, Weisshaar E, et al. Monitoring contact sensitization to p-phenylenediamine (PPD) by patch testing with PPD 0.3% in petrolatum. Contact Dermatitis. 2013; 69:26–31.

23. Spornraft-Ragaller P, Kämmerer E, Gillitzer C, Schmitt J. Severe allergic reactions to para-phenylenediamine in children and adolescents: should the patch test concentration of PPD be changed? J Dtsch Dermatol Ges. 2012; 10:258–264.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download