Abstract
Background
The scalp is frequently affected in psoriasis patients, and pruritus can adversely affect the quality of life of affected patients. Few studies have assessed pruritus in scalp psoriasis.
Objective
To determine the correlation among the clinical characteristics of pruritus, psoriasis scalp severity index (PSSI), and intraepidermal nerve fiber (IENF) density in psoriatic scalp lesions.
Methods
Eighty patients (53 men, 27 women; mean age, 46.4 years; mean PSSI, 19.9) with scalp psoriasis were evaluated by using the PSSI and the Leuven itch scale. Biopsies were obtained from the lesional and nonlesional skin of 19 patients (10 men, 9 women; mean age, 37.8 years; mean PSSI, 25.8). Immunofluorescence staining of protein gene product 9.5 was performed to determine the IENF density.
Results
Sixty-four patients (80%) complained of pruritus associated with scalp psoriasis, which negatively affected their quality of life to varying degrees. A moderate positive relation between PSSI score and pruritus intensity was identified (r=0.225 and p=0.044). The IENF density in psoriatic lesions was significantly higher than that in the nonlesional scalp (6.2±1.2 vs. 4.2±1.6, p<0.001). However, the correlations between IENF density and PSSI score, and IENF density and pruritus intensity were insignificant.
Conclusion
These results indicate that pruritus prevalence is high in patients with scalp psoriasis, and pruritus considerably influences the patients' daily lives and quality of life. In addition, high IENF density in psoriatic scalp lesions may play a role in the development of pruritus in scalp psoriasis.
Pruritus is the most common symptom of dermatologic disorders. Previous studies have reported that pruritus frequency is associated with psoriasis severity to varying degrees1,2. Many possible mediators have been suggested to transmit or modulate pruritus in psoriasis; however, the precise mechanism and the mediators involved are still unclear3. Neurogenic factors, including altered innervations, neuropeptides, opioids, and nerve growth factors (NGFs), have been implicated as important mediators in psoriatic skin4. Nakamura et al.4 reported high numbers of protein gene product 9.5 (PGP 9.5)-immunoreactive nerve fibers in the epidermis and upper dermis, and of perivascular nerve fibers containing substance P (SP) in psoriatic lesions. These findings indicate that hyperinnervation in psoriatic lesions is involved in the pathogenesis of pruritus associated with psoriasis. However, other investigators have reported conflicting results, showing decreased nerve density in psoriatic lesions compared with that in nonlesional skin5.
The scalp is the most frequently affected area in patients with psoriasis. A previous study indicated that 57% of patients with scalp psoriasis believed that it was a psychosocial handicap, and that the most annoying symptom was itching6. The scalp has a complex neuroanatomy with an abundance of sensory neural end organs in the pilosebaceous unit7. Therefore, understanding the clinical characteristics and unique features of the itch mechanism in the scalp is important for the development of effective therapies. However, few studies have focused on pruritus in scalp psoriasis.
In the present study, we investigated the clinical characteristics of pruritus associated with scalp psoriasis and examined intraepidermal nerve fiber (IENF) density in scalp psoriatic lesions. Furthermore, we evaluated the correlation among scalp psoriasis severity, IENF density, and pruritus intensity.
Patients who were at least 18 years of age and diagnosed with psoriasis with scalp involvement were eligible for the study. Those with other underlying pruritogenic diseases of the scalp, including seborrheic dermatitis, atopic dermatitis, contact dermatitis, lichen planopilaris, folliculitis, and malignancy, were excluded. Written informed consent was obtained from all patients.
The study protocol was approved by the institutional review board of Pusan National University Hospital (IRB No. D-1305-016-015), and the study was conducted according to the ethical guidelines of the Declaration of Helsinki.
We used the psoriasis scalp severity index (PSSI), which corresponds to the scalp-modified psoriasis area severity index (PASI). The resulting values were 0 to 72, analogous to the PASI values. The Leuven Itch Scale (LIS), a questionnaire that evaluates the dimensions of the itch experience, was used in this study8. All patients were asked to complete a questionnaire about the scalp. We defined the negative impact of itching as an answer of "sometimes", "often", or "always" to questions about "consequences of itching" in the LIS. The subjective mean itch intensity in the past month was evaluated by using the visual analogue scale (VAS) score: from 0 (for no itch) to 10 (for maximum unbearable itch).
Punch biopsies (4 mm diameter) were taken from the site with the most itch at the time of the biopsy in the psoriatic scalp and from the nonlesional scalp (nonlesional and nonpruritic site ≤10 mm from the site of the primary lesion) of 19 patients. Tissues were rapidly dissected, post-fixed in fixative solution (4% paraformaldehyde and 0.4% picric acid in 0.16 M sodium phosphate buffer, pH 6.9) for 90 minutes, and rinsed in 0.1 M phosphate buffer (pH 7.4) containing 10% sucrose (prepared in phosphate buffered saline [PBS]) for at least 24 hours as an optimal cutting temperature compound (Sakura Finetek Japan Co. Ltd., Tokyo, Japan). Specimens were frozen in isopentane that was cooled with liquid nitrogen and stored at -70℃. Sections were cut (25 µm) in a cryostat and air-dried overnight at room temperature. Slide-mounted tissue sections were rehydrated briefly in PBS and incubated with blocking buffer (blocking solution with goat serum; GeneTex Inc., Irvine, CA, USA) for 1 to 3 hours at room temperature. The primary antibodies used in this study were polyclonal antibodies against the neuron-specific hydrolase PGP 9.5 (Chemicon, Temecula, CA, USA). Primary antibodies were diluted in PBS to the appropriate working dilution (1:1,000). Blocking buffer was removed, and the slides were then incubated at 4℃ for 24 hours with primary antibodies. Sections were rinsed thrice in PBS and then incubated for 1 hour at room temperature with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG; Life Technologies, Carlsbad, CA, USA). Sections were then mounted in a mixture of PBS and glycerol (1:3) containing 0.1% p-phenylenediamine.
The samples were examined with a LEICA TCS SP 2 confocal microscope and measured using Leica confocal software (Leica Microsystems, Mannheim, Germany). We scanned for subepidermal and intraepidermal nerves through two planes, horizontally along the epidermaldermal border and vertically by focusing through the thickness of the section, to detect each nerve branch and its course as suggested in the published guidelines9. The length of the epidermis was measured with Olympus DP soft analySIS Image Processing (version 3.2) software. In a blinded manner, two trained researchers (TW. K and WH. S) counted the total number of IENF clearly penetrating the basement membrane in each section by following established criteria9 at ×400 magnification; however, the intraobserver and interobserver reliabilities was untested and variability was not calculated. Three specimens per biopsy were counted; the mean value was determined and divided by the epidermal length of the biopsy. The IENF density was calculated as the mean number of nerve fibers per millimeter epidermal length (IENF/mm).
Statistical analysis was performed with PASW Statistics ver. 18.0 for Windows (IBM Co., Armonk, NY, USA). Wilcoxon test was performed to compare the IENF density between lesions and nonlesions. The Spearman p test was performed to determine whether a significant correlation existed among PSSI, pruritus intensity, and IENF density. In all statistical tests, a two-sided significance test was performed and p<0.05 was considered statistically significant.
A total of 80 patients (53 men, 27 women; mean age, 46.4 years; mean PSSI, 19.9) with scalp psoriasis were enrolled in the study (Table 1). Immunoreactivity to PGP 9.5 was investigated in 19 patients. The mean VAS of pruritus was 3.81±3.06 for all patients and 5.84±2.95 for the 19 patients. There was a moderate positive relation between PSSI score and VAS of pruritus in all scalp psoriasis patients (Spearman rank correlation; r=0.225 and p=0.044) (Fig. 1). The mean VAS of distress due to itching was 4.94±3.25 for all patients and 6.0±3.43 for the 19 patients.
Sixty-four patients (80.0%) experienced pruritus associated with scalp psoriasis to varying degrees (Table 2). Of these 64 patients, 28 (35.0%) had persistent pruritus. Pruritus lasted up to 1 hour in 50 patients (62.5%), and the severity was rated as greater in the evening and at night in 38 patients (47.5%). For questions on aggravating factors of pruritus, the most common answer given by the participants was "when I was stressed out" (35.0%), followed by "in a hot environment" (18.8%), "when sweating" (17.5%), "during a change in the weather" (12.5%), and "in a cold environment" (10.0%). Fifty-four patients (67.5%) had lesion from scratching because of the pruritus, and pruritus led to a reduction in the quality of life and social contact in 42 patients (52.5%) and 22 patients (27.5%), respectively. Furthermore, pruritus associated with scalp psoriasis had a negative impact on concentration (48.8%), behavior toward others (35.0%), routine activities (35.0%), and mood (32.5%). Pruritus negatively affected sleep (5.0%) and appetite (5.0%). Of the patients, 32.5% complained of itching on the entire body, followed by the scalp and trunk (17.5%), the scalp only (16.3%), and the scalp and extremities (13.8%).
The IENF density in psoriatic lesions was significantly higher than that in the nonlesional scalp (6.2±1.2 vs. 4.2±1.6, p<0.001) (Fig. 2). IENF density showed no significant correlation with the PSSI score (Spearman rank correlation; r=-0.157 and p=0.520) and a moderate but not significant correlation with the VAS of pruritus (Spearman rank correlation; r=0.326 and p=0.173).
Several previous studies have reported pruritus associated with psoriasis in varying degrees of frequency (67%~ 84%)1,2. To our best knowledge, no study has examined pruritus associated with scalp psoriasis. The frequency of pruritus associated with scalp psoriasis was 80% in the current study, and this frequency was similar to that in previous reports of psoriasis involving skin other than the scalp. Yosipovitch et al.2 and Gupta et al.10 reported no significant relation between psoriasis severity and pruritus intensity. However, Szepietowski et al.1 showed a significant relation between psoriasis severity and pruritus intensity. In the present study, there was a moderate positive relation between scalp psoriasis severity and pruritus intensity (Spearman rank correlation; r=0.225 and p=0.044). Our results indicate that pruritus intensity may be influenced partly by the degree of inflammation through the change of pruritogenic mediators in psoriatic lesions. In addition, our study investigated pruritus limited to the scalp, which is a relatively small area, and this may have directly reflected the impact of released pruritogenic mediators. Other pruritus-influencing factors may have been excluded.
In the present study, the most aggravating factor of pruritus was stress. This supports the idea that events affecting the nervous system can influence the pruritus of psoriasis. Gupta et al.10 reported that most patients with psoriasis experienced depression to varying degrees, and that depression has a parallel course with psoriasis. Depression is known to decrease the threshold of pain and itch; thus, depressive symptoms mediated by psoriasis may be an important cause of pruritus11. Bondy et al.12 showed that patients with major depression have increased level of serum SP and thus might indicate a subgroup of the disorder in which neuropeptides have a key role. In this respect, SP is considered the crucial neuropeptide in psoriasis and pruritus, and an alteration of SP levels results in an exacerbation of psoriasis and pruritus12,13,14.
In the literature, pruritus associated with scalp psoriasis negatively affects quality of life to varying degrees. In a survey conducted by the National Psoriasis Foundation, almost 75% of patients believed that psoriasis had a moderate to large negative impact on their quality of life, with alterations in their daily activities15. Similarly, pruritus associated with the scalp commonly leads to unwanted and unpleasant social consequences. A recent survey reported that almost half of patients with psoriasis experienced sleep interference at least once a month because of psoriasis16. In this study, only four patients complained of sleep disturbance or decreased appetite. However, all four patients had high-intensity itch; thus, pruritus intensity may affect sleep or appetite. Most psoriasis patients with pruritic scalp also have psoriatic lesions with variable intensities of itch on other sites of the body. We believe that further studies may be required to characterize the differences in itch intensity according to the involved body regions.
Our study showed that the IENF density of the psoriatic scalp was significantly higher than that of the nonlesional scalp. Other studies of PGP 9.5-immunoreactive nerve fibers in psoriatic skin have reported conflicting results. Taneda et al.17 showed that the number of epidermal nerve fibers was increased in approximately 40% of psoriatic patients with pruritus compared with the number in healthy controls. In contrast, Johansson et al.18 reported a profound reduction in the epidermal nerve fiber density in psoriatic skin. Our immunohistochemical data support previous studies that described hyperinnervation in psoriatic lesions. The pathogenesis of the alteration of innervations in psoriatic lesions is thought to result secondary to inflammatory reactions in the dermis with the more prominent development of dermal papillary blood vessels and acceleration of the sprouting of cutaneous nerve caused by NGFs in psoriatic patients with pruritus19,20. Our results did no t show a significant correlation among IENF density in lesional skin, PSSI (p=0.520), and pruritus intensity on VAS (p=0.173). Cutaneous innervations in psoriatic lesions may be associated with the degree of skin inflammation and other pruritogenic factors, including epidermal barrier abnormality, and psychological and environmental conditions. Many inflammatory mediators may be involved in itch development. Further, the scalp has a complex neuroanatomy with an abundance of sensory neural end organs in the pilosebaceous unit. Thus, the pathogenesis of itch in scalp psoriasis can differ from psoriasis in other body sites7.
The small sample size in the nerve stain group is a limitation of this study, and the selection bias could not be ignored. Additionally, although both observers investigated IENF density in a blinded manner, the interobserver and intraobserver reliabilities were untested and variability was not calculated. To more clearly establish the role of IENF density in pruritus of scalp psoriasis, it might be necessary to compare the IENF of itching and non-itching psoriatic scalp lesions. Further, it was difficult to evaluate the influence of scalp pruritus on the quality of life and clinical characteristics of patients with pruritus of the scalp and of other locations.
In conclusion, the prevalence of pruritus was high among patients with scalp psoriasis, and pruritus had a considerable impact on patients' daily living and quality of life. In addition, IENF density was significantly increased in the psoriatic scalp as compared with its density in the nonlesional scalp. Therefore, neurogenic factors, including increased IENF density in psoriatic scalp lesions, may play a role in the development of pruritus.
Figures and Tables
 | Fig. 1Correlation between psoriasis scalp severity index (PSSI) score and intensity of pruritus (visual analogue scale [VAS]) (r=0.225, p=0.044). |
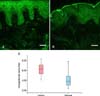 | Fig. 2Immunofluorescence micrographs of psoriatic (A) and nonlesional (B) scalp after incubation with antiserum to protein gene product 9.5 (PGP 9.5). Scale bar=50 µm. (C) Semiquantitative analysis of the number of PGP 9.5-immunoreactive nerve fibers clearly penetrating the basement membrane. |
References
1. Szepietowski JC, Reich A, Wi . 347;nicka B. Itching in patients suffering from psoriasis. Acta Dermatovenerol Croat. 2002; 10:221–226.
2. Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000; 143:969–973.

3. Reich A, Szepietowski JC. Mediators of pruritus in psoriasis. Mediators Inflamm. 2007; 2007:64727.

4. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003; 149:718–730.

5. Pergolizzi S, Vaccaro M, Magaudda L, Mondello MR, Arco A, Bramanti P, et al. Immunohistochemical study of epidermal nerve fibres in involved and uninvolved psoriatic skin using confocal laser scanning microscopy. Arch Dermatol Res. 1998; 290:483–489.

6. van de Kerkhof PC, de Hoop D, de Korte J, Kuipers MV. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology. 1998; 197:326–334.

7. Bin Saif GA, Ericson ME, Yosipovitch G. The itchy scalp-scratching for an explanation. Exp Dermatol. 2011; 20:959–968.
8. Haest C, Casaer MP, Daems A, De Vos B, Vermeersch E, Morren MA, et al. Measurement of itching: validation of the Leuven Itch Scale. Burns. 2011; 37:939–950.

9. Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. European Federation of Neurological Societies. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005; 12:747–758.

10. Gupta MA, Gupta AK, Kirkby S, Weiner HK, Mace TM, Schork NJ, et al. Pruritus in psoriasis. A prospective study of some psychiatric and dermatologic correlates. Arch Dermatol. 1988; 124:1052–1057.

11. Gupta MA. Is chronic pain a variant of depressive illness? A critical review. Can J Psychiatry. 1986; 31:241–248.

12. Bondy B, Baghai TC, Minov C, Schüle C, Schwarz MJ, Zwanzger P, et al. Substance P serum levels are increased in major depression: preliminary results. Biol Psychiatry. 2003; 53:538–542.

13. Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978; 71:233–235.

14. Ellis CN, Berberian B, Sulica VI, Dodd WA, Jarratt MT, Katz HI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993; 29:438–442.

15. Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001; 137:280–284.
16. Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009; 60:604–608.

17. Taneda K, Tominaga M, Negi O, Tengara S, Kamo A, Ogawa H, et al. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011; 165:277–284.

18. Johansson O, Han SW, Enhamre A. Altered cutaneous innervation in psoriatic skin as revealed by PGP 9.5 immunohistochemistry. Arch Dermatol Res. 1991; 283:519–523.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download