Abstract
Background
Certain epidermal appendage tumors, including hyperplasias (hamartomas), adenomas, benign epitheliomas, primordial epitheliomas, and malignant tumors, can exhibit any stage of differentiation. Several molecules associated with tumorigenesis, such as Gli-1, pleckstrin homology-like domain, family A, member 1 (PHLDA-1), transforming growth factor (TGF)-β1, TGF-β2, and p63, are associated with tumor grade and aggressive behavior in follicular and sebaceous tumors in ways that are not well understood.
Objective
The aim of this study was to elucidate the expression of Gli-1, PHLDA-1, TGF-β1/β2, and p63 in benign and malignant tumors of the hair and sebaceous glands and to determine their importance in the degree of tumor differentiation.
Methods
Immunohistochemistry was performed in follicular and sebaceous tumors using antibodies against Gli-1 (sebaceous tumor marker), PHLDA-1 (hair follicle outer root sheath [ORS] cell marker), p63, TGF-β1, and TGF-β2.
Results
Gli-1 was expressed in basaloid cells, sebocytes, and sebaceous carcinoma cells, and expression levels decreased as differentiation progressed. PHLDA-1 was expressed in ORS cells and some follicular tumor cells. Expression of p63 was observed in the nuclei of the outermost basaloid cells (seboblasts), poorly differentiated sebaceous carcinoma cells, and tumor cells toward the direction of the hair. Remarkably, TGF-β1 was expressed exclusively in the nuclei of benign and malignant follicular (hair) tumors, but not in sebaceous tumors, at levels that correlated with the degree of differentiation.
Epidermal stem cells located in the bulge region are responsible for replenishing the hair lineage, while stem cells in the isthmus (the junction between the infundibulum and hair follicle) regenerate the interfollicular epidermis and sebaceous glands (SGs)1,2,3,4.
Epidermal appendage tumors, such as hyperplasias (hamartomas), adenomas, benign epitheliomas, primordial epitheliomas, and malignant tumors, may exhibit specific stages of differentiation. Sebaceous adenoma arises from the upper region of the hair follicle and consists of a proliferative outer layer of keratinocytes and an inner differentiation compartment of sebocytes5. Trichofolliculomas show evidence of differentiation towards the hair shaft and inner root sheath lineages5.
Human Gli-1 is a developmental transcription activator that is upregulated in numerous tumors, including muscle and brain tumors (leiomyomas and gliomas) and basal cell carcinoma (BCC). Gli-1 expression levels correlate with tumor grade in bone and soft tissue sarcoma6. A study by Niemann et al.7 found that Indian hedgehog (IHH) was expressed in differentiated sebocytes in normal SGs, while Gli-1 was activated in sebocyte progenitors, suggesting a paracrine signaling mechanism.
Pleckstrin homology-like domain, family A, member 1 (PHLDA-1) is a marker of hair follicle outer root sheath (ORS) cells that is prominently expressed in the hair follicle bulge of terminal and vellus hair follicles8. According to a study by Sellheyer and Krahl9, PHLDA-1 distinguishes between desmoplastic trichoepithelioma and non-ulcerated morpheic BCC.
Expression of the catagen-stage hair follicle marker transforming growth factor (TGF)-β1 is increased in the ORS and epithelial strands of the hair. Recently, it has been suggested that TGF-β signaling plays an important role in the regulation of proliferation, differentiation, and apoptosis of distinct epithelial stem cell populations in hair follicles10. The tumor suppressors TGF-β1 and TGF-β2 may also have pro-oncogenic functions during the carcinogenesis process10,11, although the exact nature of the complex roles of these molecules is not fully understood.
In some ways, neoplastic transformation may recapitulate the developmental stages of embryonic cells. However, it is currently unclear whether expression levels of the previously mentioned molecules correlate with tumor grade in sebaceous and follicular lineage tumors. In addition, the clinical usefulness of these molecules in sebaceous and follicular tumors is not well established.
Therefore, in this study, we evaluated the expression levels and localization patterns of Gli-1, PHLDA-1, p63, TGF-β1, and TGF-β2 in various sebaceous and follicular tumors.
The institutional review boards of Dong-A University Hospital in Busan, Korea approved this study, and this study was performed in accordance with the guidelines of the Declaration of Helsinki. Archival materials were obtained between 2002 and 2011. Information about the diagnosis of skin adnexal tumors, such as whether they were hyperplastic (hamartomatous), benign, or malignant sebaceous or follicular tumors, was retrieved from the Dong-A University Hospital database. A total of 71 cases with available archival materials were retrieved from the pathology files of the Departments of Dermatology and Pathology. To confirm the diagnoses, 2 dermatologists independently reviewed the histological sections from all cases. After review, 18 sebaceous tumors and 16 follicular tumors were selected for this study (Table 1). Clinical information was extracted from medical records. All patient data were de-identified.
Immunohistochemical staining for Gli-1, PHLDA-1, TGF-β1, and TGF-β2 was performed on formalin-fixed, paraffin-embedded sections. Sections were cut to be 4-µm thick and were mounted on gelatin-coated slides. Slides were heated at 60℃ for 12 h, deparaffinized in xylene, and rehydrated in a graded series of alcohol. For antigen retrieval, the slides were dipped in 0.01 M citric acid buffer and boiled in a microwave for 4 min. The procedure was repeated 4 times, in cycles of 30 s of boiling and 30 s of cooling in a microwave. The tissues were left to cool for 1 h at room temperature, and then processed with 3% hydrogen peroxide for 5 min at room temperature to inactivate endogenous peroxidase. After reacting for 1 h at room temperature, slides were incubated with primary antibodies for Gli-1, PHLDA-1, TGF-β1, and TGF-β2 and allowed to react with the streptavidin horseradish peroxidase complex at room temperature for 30 min (Table 2). Thereafter, a color reaction was performed using 100 µl Dako substrate buffer and 2 µl DAB (DAKO Corp., Carpinteria, CA, USA). The reaction time depended on the state of tissues. The slides were examined after washing with hematoxylin as the contrast stain.
For evaluation of p63 expression patterns, paraffin-embedded tissue blocks were cut and processed in the manner described above. Tissue sections were stained using an automatic immunohistochemical stainer (Bench Mark XT; Ventana Medical Systems Inc., Tucson, AZ, USA). Immunohistochemical staining was performed as recommended by the manufacturer (Ventana Medical Systems Inc.); the automatic stainer programs included (1) deparaffinization, (2) rehydration, (3) heat-induced antigen retrieval (42℃, 30 min), and (4) detection with the Ultra-View Universal DAB Detection Kit (Ventana Medical Systems Inc.).
We investigated the intensity and localization patterns of staining in tumors and normal skin epidermal and dermal components by assessing slides from at least 3 dishes. Slides were classified into the following categories according to the staining intensity of the previously mentioned factors: absence of any staining (0), minimal intensity (1), mild intensity (2), moderate intensity (3), and maximal intensity (4). We also categorized the staining as nuclear, cytoplasmic, or nuclear and cytoplasmic according to the localization pattern of the staining. Two dermatologists independently evaluated the immunohistochemical results. The dermatologists reviewed any disagreements together to reach a consensus score. For evaluation of the objective intensity, human glioma (for Gli-1) and normal skin (normal follicular bulge for PHLDA-1 and basal layer of the epidermis for TGF-β1, TGF-β2, and p63) tissues were used as positive controls because they were easily available and had relatively stable reactivity. The primary antibody was replaced with phosphate-buffered saline for negative controls.
Gli-1 was highly expressed in the basaloid cells (progenitor cells) and premature sebocytes in benign and hyperplastic sebaceous tumors, and its expression was decreased in well-differentiated (mature) sebocytes (Fig. 1). Gli-1 was highly expressed in poorly differentiated sebaceous carcinoma cells. In contrast, Gli-1 expression was decreased in well-differentiated sebaceous carcinoma cells (Fig. 1, Table 3). Although Gli-1 was expressed in the nuclei and cytoplasms of morpheic BCCs, Gli-1 expression was not observed in other follicular tumors.
PHLDA-1 was highly expressed in the ORS and peripheral tumor cells of benign and hyperplastic follicular tumors. PHLDA-1 expression was decreased in the cells of other hair follicle layers and at the center of tumors (Fig. 2). High expression of PHLDA-1 was observed in poorly differentiated trichilemmal carcinoma cells, and expression was decreased in the most well-differentiated trichilemmal carcinoma cells (Fig. 2, Table 4). Expression of PHLDA-1 was not observed in morpheic BCCs or any sebaceous tumors.
TGF-β1 was expressed only in the nuclei of basaloid cells in benign and hyperplastic sebaceous tumors. Although TGF-β1 expression was observed in normal keratinocytes in the lower portion of the epidermis, it was not expressed in any sebaceous carcinoma specimens (Fig. 3, Table 5). TGF-β1 was highly expressed in the nuclei of ORS and peripheral tumor cells in benign and hyperplastic follicular tumors, which was in contrast to its decreased expression in the cells of other hair follicle layers and the center of tumors (Fig. 3). TGF-β1 was also highly expressed in the nuclei of well-differentiated trichilemmal carcinoma cells, although its expression was decreased in the most poorly differentiated trichilemmal carcinoma cells (Fig. 3, Table 5).
TGF-β2 was expressed in the nuclei and cytoplasms of normal keratinocytes in the lower portion of the epidermis. However, TGF-β2 was not expressed in follicular and sebaceous tumor cells (Fig. 4). TGF-β2 expression was only observed in the stromal cells of the peritumoral regions of follicular tumors (Fig. 4E, F).
Expression of p63 was predominantly observed in the nuclei of basaloid cells (progenitor cells) and premature sebocytes in hyperplastic and benign sebaceous tumors. However, p63 expression was decreased in the more well-differentiated (mature) sebocytes (Fig. 1). Expression of p63 was mainly observed in poorly differentiated sebaceous carcinoma, and its expression was decreased in the most well-differentiated sebaceous carcinoma (Fig. 1, Table 4).
In benign follicular tumors, p63 was highly expressed in the nuclei of ORS and peripheral tumor cells. However, p63 expression was decreased in the central region of tumors (Fig. 2, Table 4). Similar to the observations in sebaceous carcinomas, p63 was predominantly expressed in the nuclei of poorly differentiated trichilemmal carcinomas, and its expression was decreased in the most well-differentiated trichilemmal carcinoma (Fig. 2, Table 3).
Epidermal stem cells are common targets for neoplastic conversion, and the range of epithelial tumor types reflects aberrant differentiation in different epidermal lineages. Many molecules that regulate epidermal self-renewal and differentiation have been identified2,3. However, to our knowledge, no studies have examined the molecules that regulate tumorigenesis in follicular and sebaceous tumors. A previous study found that IHH was expressed in differentiated sebocytes in normal SGs, and downstream Gli-1 is also activated in sebocyte progenitors7. Consistent with this previous study, we found that Gli-1 was expressed in normal SGs and sebaceous lineage tumors. Furthermore, Gli-1 expression was lower in well-differentiated (mature) sebocytes. However, Gli-1 expression was not observed in any follicular tumors, except for morphemic BCCs. Therefore, we suggest that Gli-1 is negatively associated with sebocyte maturity and maybe a useful marker of sebaceous tumors. A previous study demonstrated that p53 negatively regulates Gli-1 in multiple systems, and Gli-1 and p53 form a novel homeostatic inhibitory loop that normally controls precursor and stem cell numbers12. The study also found that imbalances in favor of Gli-1 are prevalent in cancer and cancer stem cells. For example, glioma cancer stem cells require sustained IHH-Gli function13, and gliomas frequently lack p5314. Our study also found that poorly differentiated sebaceous carcinoma had increased Gli-1 expression compared to mature sebocytes and well-differentiated sebaceous carcinoma. In terms of biological behavior, these data indicate that Gli-1 expression is highly associated with the degree of differentiation of benign and malignant sebocytes.
PHLDA-1 is prominently expressed in the hair follicle bulge and may be a useful marker of bulge stem cells8. In this study, we found that PHLDA-1 was highly expressed in the ORS and peripheral tumor cells in benign and hyperplastic follicular tumors. Additionally, PHLDA-1 expression was decreased in the cells of other hair follicle layers and in cells in the central region of tumors. However, PHLDA-1 was not expressed in any sebaceous tumors. Therefore, we suggest that PHLDA-1 may correlate with follicular differentiation and be a useful marker for hair follicle tumors. In our study, PHLDA-1 was highly expressed in poorly differentiated trichilemmal carcinoma cells, and its expression was decreased in the most well-differentiated trichilemmal carcinoma cells. Therefore, it is likely that PHLDA-1 expression is positively associated with the aggressiveness of follicular tumors.
The important role of p63 in cell fate specification, cell proliferation, differentiation, senescence, and adhesion is well accepted15,16. Collectively, published data suggest that p63 functions as an oncogene rather than a tumor suppressor gene. A past report indicated that increased p63 expression is associated with a more aggressive phenotype and poor prognosis in oral squamous cell carcinoma (SCC)17. Similarly, another previous study found an association between high p63 expression in SCC and aggressive behavior and poorprognosis18. Several previous studies evaluated p63 expression in cutaneous adnexal tumors, and most reports indicated that p63 may be a marker of basal and progenitor cells in tumors of the epidermis and epidermal appendages, and may be a diagnostic marker for these tumors19,20,21,22. To better understand the role of p63 in carcinogenesis, it is important to determine the variant that is expressed in follicular and sebaceous tumors. We evaluated p63 expression in these tumors and demonstrated that p63 was predominantly expressed in the nuclei of peripheral tumor cells and poorly differentiated carcinoma cells. Therefore, we suggest that p63 expression may play a role in the differentiation and carcinogenesis (aggressive biological behavior) of sebaceous and follicular tumors. Furthermore, we propose that p63 is a useful marker for determining the degree of differentiation and malignant potential of sebaceous and follicular tumors.
It is generally accepted that TGF-β signaling has multiple roles in epithelial cells, which are the cells that give rise to the majority of human cancers. TGF-β functions range from cell growth arrest to enhancement of migration and initiation of cell morphology changes. A recent study demonstrated that TGF-β has biphasic effects during tumorigenesis; it acts as a tumor suppressor in early stage cancer, and stimulates cancer progression via its action on tumor cells and their microenvironment in later stages23. Furthermore, previous studies showed that TGF-β expression levels are positively associated with aggressive biological behavior in tumors24. Our data show a negative association between TGF-β1 expression and aggressive biological behavior of follicular tumors, although the follicular organoid differentiation was more conspicuous in benign and hyperplastic follicular tumors than in trichilemmal carcinoma. Adorno et al.25 reported that, in cells that expressed mutant p63, the mutant p53-Smad complex inactivates transcriptional activity, which is induced by TGF-β, and that p63 acts as an antagonist of TGF-β in tumor invasiveness and metastasis. Therefore, we assume that the negative association between TGF-β1 and p63 expression might be related to the antagonistic effects of both molecules. Based on our results, we suggest that TGF-β1 may also be useful for determining the degree of differentiation and malignant potential of hair follicle tumors. Furthermore, TGF-β1 expression was not observed in any sebaceous tumors. We suggest that TGF-β1 may be a useful marker for distinguishing between trichilemmal and sebaceous carcinoma.
When we examined tumorigenesis in follicular tumors and sebaceous tumors, we found that TGF-β2 was not expressed in either tumor lineage. TGF-β2 was only expressed in stromal cells of the peritumoral area. TGF-β2 is essential for induction of the catagen phase of the human hair cycle26. However, a recent study suggested that dermal papilla cells (DPCs) express TGF-β2 and that it plays an important role in hair follicle morphogenesis27. Therefore, the TGF-β2-positive stromal cells that we observed were likely DPCs and may be important in the tumorigenesis of follicular tumors.
We acknowledge that the scope of this study was limited. The number of samples selected was small. Furthermore, we only performed immunohistochemistry tests and did not perform western blots or enzyme-linked immunoassays to quantify the expression of these molecules. Therefore, we could not perform statistical analysis or confirm that the results of our study are conclusive. Consequently, we suggest that future quantitative studies with large sample sizes are needed to confirm these preliminary results.
To our knowledge, this preliminary study is the first to evaluate the expression of Gli-1, PHLDA-1, TGF-β1, TGF-β2, and p63 in benign and malignant tumors of the hair and SGs and to determine their importance in the degree of differentiation of these tumors. Our results indicate that Gli-1 and PHLDA-1 may be useful markers of sebaceous and follicular tumors, respectively. In terms of tumorigenesis, we found that p63 expression may be useful for determining the degree of differentiation and the malignant potential of sebaceous and follicular tumors. TGF-β1 expression may be useful in determining the degree of differentiation and malignant potential of hair follicle tumors. Furthermore, we suggest that TGF-β1 may be a useful marker for distinguishing between trichilemmal and sebaceous carcinomas.
Figures and Tables
Fig. 1
Hematoxylin and eosin (H&E) staining and Gli-1 and p63 immunohistochemistry in sebaceous neoplasms. (A, D, G) H&E, (B, E, H) Gli-1, (C, F, I) p63, (J, K) positive controls, and (L, M) negative controls.
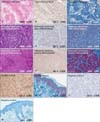
Fig. 2
Hematoxylin and eosin (H&E) stains and pleckstrin homology-like domain, family A, member 1 (PHLDA-1) and p63 immunohistochemistry infollicular neoplasms. (A, D, G) H&E, (B, E, H) PHLDA-1, (C, F, I) p63, (J) positive control, and (K) negative control.
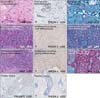
Fig. 3
Transforming growth factor-β1 immunohistochemistry in sebaceous and follicular tumors. (A~C) Sebaceous tumors, (E~G) follicular tumors, (D) positive control, and (H) negative control.

Fig. 4
Transforming growth factor-β2 (TGF-β2) immunohistochemistry insebaceous and follicular tumors. (A~C) Sebaceous tumors, (E~G) follicular tumors, (D) positive control, and (H) negative control. Arrows: TGF-β2 expression.

References
1. Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001; 114:3419–3431.

2. Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002; 12:185–192.

3. Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002; 3:199–209.

4. Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000; 102:451–461.

5. Turusov VS, Mohr U. Pathology of tumours in laboratory animals. Volume II, tumours of the mouse. Lyon, France: International Agency for Research on Cancer;1994. p. 1–26.
6. Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000; 67:1047–1054.

7. Niemann C, Unden AB, Lyle S, Zouboulis ChC, Toftgård R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003; 100:Suppl 1. 11873–11880.

8. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006; 116:249–260.

9. Sellheyer K, Krahl D. PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. Br J Dermatol. 2011; 164:141–147.

10. Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelialmesenchymal transition. Curr Opin Cell Biol. 2009; 21:166–176.

11. Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002; 12:22–29.
12. Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009; 28:663–676.

13. Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007; 17:165–172.

14. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007; 170:1445–1453.

15. Viganò MA, Mantovani R. Hitting the numbers: the emerging network of p63 targets. Cell Cycle. 2007; 6:233–239.

16. Perez CA, Pietenpol JA. Transcriptional programs regulated by p63 in normal epithelium and tumors. Cell Cycle. 2007; 6:246–254.

17. Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol. 2005; 36:187–194.

18. Gu X, Coates PJ, Boldrup L, Nylander K. p63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett. 2008; 263:26–34.

19. Tsujita-Kyutoku M, Kiuchi K, Danbara N, Yuri T, Senzaki H, Tsubura A. p63 expression in normal human epidermis and epidermal appendages and their tumors. J Cutan Pathol. 2003; 30:11–17.

20. Qureshi HS, Ormsby AH, Lee MW, Zarbo RJ, Ma CK. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004; 31:145–152.

21. Plaza JA, Ortega PF, Stockman DL, Suster S. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol. 2010; 37:403–410.

22. Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010; 23:713–719.

23. Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001; 11:S44–S51.
24. Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013; 25:76–84.

25. Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009; 137:87–98.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download