Abstract
Anetoderma is a rare cutaneous disorder characterized by a loss of normal elastic tissue that presents clinically as atrophic patches located mainly on the upper trunk. Recent studies suggest immunological mechanisms may play a role in this process. Furthermore, a secondary form of macular atrophy occurs in the course of infectious diseases (e.g. syphilis and tuberculosis) and autoimmune disease (e.g. systemic lupus erythematosus [SLE]). Here, we report the case of a 20-year-old woman previously diagnosed with SLE, who presented with numerous well-circumscribed atrophic macules on the face and upper trunk. Histopathological examination showed decreased elastic tissues in the reticular dermis and mononuclear cells adhering to elastic fibers, consistent with anetoderma. Thus, the eruptive anetoderma localized widely on the face and upper trunk may have been caused by an autoimmune response of SLE.
Anetoderma is an uncommon cutaneous disease characterized by a decrease in the amount of normal elastic tissue. However, its precise pathophysiology remains unclear. Clinically, anetoderma manifests as localized laxity of the skin with herniation or out-pouching resulting from abnormal dermal elastic tissue1. The associations of this condition with several immunological abnormalities such as systemic lupus erythematosus (SLE) and primary hypothyroidism with anti-thyroid antibodies as well as several infectious diseases such as syphilis and tuberculosis suggests an immunological mechanism may play a role in the elastolytic process in anetoderma2,3. There are few case reports regarding anetoderma or cutis laxa-like lesions associated with SLE. Here, we report the case of a female SLE patient with eruptive anetoderma spreading gradually on the face and upper trunk. The observations in this case may help explain the immunological pathogenesis of anetoderma in SLE.
A 20-year-old woman presented with a 1-year history of gradually spreading white atrophic macules on the face and upper trunk. She had been diagnosed with SLE 3 years previously and was treated with hydroxychloroquine sulfate. Blood chemistry showed antiphospholipid, anticardiolipin, and lupus anticoagulant antibodies were all normal while complement levels were slightly low. The lesions were multiple 1~2-cm shiny white atrophic macules on the face and upper trunk (Fig. 1). Histopathological examination of the back showed atrophic epidermis and marked decreases in the amount of elastic fibers in the upper dermis and mononuclear cells adhering to elastic fibers. Some thrombosis of the small vessels was observed, but there were no signs of vasculitis (Fig. 2A, B). Elastic staining revealed a loss of elastic fibers, which was more evident in the upper dermis (Fig. 2C). Biopsy confirmed a diagnosis of anetoderma, which was hypothesized to be due to an autoimmune disorder. The patient is still receiving treatment for SLE with oral hydroxychloroquine sulfate and antihistamine, but the skin lesions have shown little improvement. As SLE progressed, the skin lesions exhibited aggravation.
Primary anetoderma is an idiopathic condition and can also be seen in association with autoimmune diseases such as SLE or primary hypothyroidism, occurring when there are no underlying skin diseases. Secondary anetoderma implies characteristic atrophic lesions appearing at the same sites where specific dermatoses such as acne or varicella lesions have previously occurred4. An ultrastructural study investigated the changes in elastic fibers in the cutaneous lesions of 7 patients with LE; the results show a loss of dermal elastin matrix, resulting in a relative increase in the predominance of elastofibrils and the formation of what are termed "dermal fibrillar bodies"5. The LE-associated loss of elastic fibers has been reported in lesions similar to anetoderma. Unlike the white firm non-follicular papules in papular elastorrhexis, depressed atrophic lesions are the main feature of anetoderma. Moreover, it can be distinguished from extragenital lichen sclerosus because it clinically manifests as a porcelain white atrophic plaque4. Although the causative mechanism of anetodermic lesions is unknown, it may be due to microthrombosis in the dermal vessels, inducing the development of local ischemia and leading to elastic tissue degeneration6,7. No association between primary anetoderma and SLE has been identified, but the findings of microthrombosis in some skin biopsies and in some cases hypocomplementemia, hypergammaglobulinemia, or circulating antiphospholipid antibodies (e.g. lupus anticoagulant antibody) can cause ischemia of dermal tissues and trigger elastic fiber degeneration8,9. On the other hand, there appears to be a correlation between increased anticardiolipin antibody levels and the development of cutaneous lesions of anetoderma in human immunodeficiency virus type 1 (HIV-1) disease. Anetoderma may occur in patients with HIV infection, and anticardiolipin antibodies may alter the function of dermal proteinases, thereby destroying elastic fibers10. However, the present case was negative for antiphospholipid and anticardiolipin antibodies, although there were mild decreases in complement levels and D-dimer level was elevated. Therefore, we postulate that the spread of anetodermic lesions in the present patient was mediated by microthromboses in the dermal vessels, resulting in the development of local ischemia and degeneration of elastic tissue due to the immunological response in SLE. In addition, as the anetodermic lesions were aggravated following the clinical progression of SLE, we considered the anetoderma to have occurred in association with SLE. Further data in clinical cases of SLE with anetoderma must be collected to elucidate the pathophysiology of anetoderma in SLE patients. Because of the possible clinical relevance of the association between primary anetoderma and SLE, we recommend that patients with anetoderma be examined for antiphospholipid antibodies or other autoimmune antibodies as well as for hypercoagulable states.
Figures and Tables
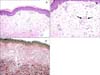
Fig. 2
(A) Atrophic epidermis and perivascular lymphohistiocytic infiltration in upper dermis (H&E, ×100). (B) The small vessels of the upper dermis progress toward thrombosis (arrows) without signs of vasculitis (H&E, ×200). (C) Elastic stain revealed loss of elastic fibers, which was more evident in the upper dermis (elastin, ×100).
ACKNOWLEDGMENT
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN10C0013).
References
1. Venencie PY, Winkelmann RK. Monoclonal antibody studies in the skin lesions of patients with anetoderma. Arch Dermatol. 1985; 121:747–749.

2. Hodak E, Shamai-Lubovitz O, David M, Hazaz B, Katzenelson-Weissman V, Lahav M, et al. Immunologic abnormalities associated with primary anetoderma. Arch Dermatol. 1992; 128:799–803.

3. Romaní J, Pérez F, Llobet M, Planagumá M, Pujol RM. Anetoderma associated with antiphospholipid antibodies: case report and review of the literature. J Eur Acad Dermatol Venereol. 2001; 15:175–178.

4. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill;2008. p. 547p. 567–568.
5. Schmitt D, Thivolet J, Perrot H. Ultrastructural study of the cutaneous elastic fibres in lupus erythematosus. Br J Dermatol. 1972; 87:355–360.

6. Sparsa A, Piette JC, Wechsler B, Amoura Z, Francès C. Anetoderma and its prothrombotic abnormalities. J Am Acad Dermatol. 2003; 49:1008–1012.

7. Hodak E, David M. Primary anetoderma and antiphospholipid antibodies--review of the literature. Clin Rev Allergy Immunol. 2007; 32:162–166.

8. Hodak E, Shamai-Lubovitz O, David M, Hazaz B, Lahav M, Sandbank M. Primary anetoderma associated with a wide spectrum of autoimmune abnormalities. J Am Acad Dermatol. 1991; 25:415–418.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download