Abstract
Background
Among the various types of folliculitis, differentiation of Malassezia folliculitis (MF) from other forms of folliculitis is important because it is usually treated with antifungal agents.
Objective
We attempted to find a method to enhance the detection rate of MF, and examined the differences in the clinical manifestation between MF and non-MF (NMF).
Methods
We performed a retrospective study involving patients with folliculitis who were previously diagnosed with MF or NMF on the basis of serial tissue sectioning and diastase-Periodic acid-Schiff (d-PAS) staining findings. The clinical features of MF and NMF were compared.
Results
Among a total of 100 folliculitis patients, 20 were diagnosed with MF and 80 with NMF. Tissues from the 80 patients with NMF were sectioned serially into 10 slices and stained with hematoxylin and eosin stain; among these, 10 had many round-to-oval yeast organisms in the hair follicles that confirmed MF. Finally, d-PAS staining was used to detect the presence of yeast in the NMF slides. Notably, among the 70 d-PAS-stained samples, yeast organisms were found in 6 samples, confirming MF. As a result, the diagnosis of 16 patients changed from NMF to MF. Compared with NMF, MF showed major involvement of the trunk and low involvement of the face and legs as well as male predilection.
Folliculitis is an inflammatory disorder involving the superficial or deep portion of the hair follicles. The clinical manifestations include erythematous pustules or papulopustules in the acute phase1. The various causes of folliculitis range frominfection with bacterial, viral, and fungal organismstoother noninfectious causes2. Common histopathologic findings are follicular inflammatory cell infiltration with neutrophils, lymphocytes, and sometimes eosinophils, the proportions of which depend on the origin of the folliculitis. Malassezia folliculitis (MF), a form of folliculitis caused by yeast infection, is characterized by dome-shaped papules, pustules, nodules, and cysts in severe cases. Inflammatory infiltrates consisting of lymphocytes and neutrophils with focal ruptured follicles can be observed in the histopathologic analysis of MF skin samples3. Although MF and non-MF (NMF) exhibit common clinical and histopathologic findings, it is important to differentiate between the 2 conditions because their treatments tend to differ. For the treatment of MF, antifungal agents are used rather than antibiotics or corticosteroids3. Based on these factors, we reviewed previous cases of folliculitis in which patients were diagnosed on the basis of the findings of serial sections of tissue block and histochemical staining for identifying undetected Malassezia species. In addition, we examined the differences in the clinical manifestations between MF and NMF.
We collected and reviewed cases of folliculitis where the diagnosis was madeon the basis of skin biopsy findings at our institution from 2008 to 2011. Clinical information was retrospectively obtained through review of medical charts, and histologic information was obtained through slides stained with hematoxylin and eosin (H&E). We divided the cases of folliculitis into MF and NMF. MF was confirmed on the basis of pathological findings of follicular inflammatory infiltration with abundant round yeast cells in the follicles and/or perifollicular dermis. In contrast, NMF was defined as follicular infiltration of inflammatory cells including neutrophils without the definite presence of yeast cells.
In the histopathologic evaluation, the first step was serial section for visualizing hair follicles and follicular yeast cells in patients initially diagnosed with NMF. Tissues were serially sectioned from formalin-fixed paraffin blocksinto 10 slices with a thickness of 0.5 µm each. After serial section, 2 dermatopathologists confirmed the presence of inflammation in all hair follicle slides. In addition, they ascertained the presence of yeast cells in the hair follicles or perifollicular dermis, leading to a change in the diagnosis to MF. The second step of the evaluation was a histochemical study using diastase-Periodic acid-Schiff (d-PAS) stain for the remaining NMF cases to identify undetected yeast cells, with diastase-resistant cells staining red.
After evaluating and dividing the cases into MF and NMF, a retrospective chart review was performed to compare their clinical features. Various data were obtained from the medical charts: sex, age, duration and distribution of symptoms, and clinical morphology of the skin lesions. In addition to chart review, the distribution and clinical morphology of the skin lesions were visualized and evaluated using clinical photographs taken before the skin biopsy. Lesion distribution was divided according to 5 areas of the body (face, scalp, trunk, arm, and leg). Concerning clinical morphology, the skin lesions were divided into papules, pustules, and papulopustules in cases of mixed skin lesions.
Statistical analysis of the collected clinical data wasperformed using the t-test or the Fisher's exact test. Data were expressed as mean values with standard deviation. A p-value of <0.05 was considered statistically significant. IBM SPSS Statistics 20.0 (IBM Co., Armonk, NY, USA) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) were used for data analysis. The study protocol was approved by the Institutional Review Board of our institution (IRB No. MED-KSP-12-424).
We studied the cases of 100 patients previously diagnosed with folliculitis (Fig. 1). Among them, 20 patients were diagnosed with MF: 11 of the 20 patients with MF showed poor response to treatment with conventional folliculitis medication, such as minocycline; therefore, they revisited our institution; furthermore, 17 of 20 patients showed clinical improvement after receiving oral and/or topical antifungal medication, such as itraconazole, fluconazole, after histologic confirmation of the folliculitis as MF. The remaining 80 cases were diagnosed as NMF. Serial section with H&E stain was performed for these 80 patients, and follicular infiltration of inflammatory cells was visualized precisely in 10 cases with abundant eosinophilic round-to-oval yeast cells in the follicles (Fig. 2). As a result, the diagnosis in these 10 cases changed from NMF to MF. Among the remaining 70 cases of NMF for which d-PAS staining was performed, the samples from 6 patients were PAS-positive and diastase-negative, confirming the diagnosis of MF (Fig. 3). Therefore, the diagnosis of 16 cases of NMF changed to MF finally. Then, we divided these cases into 2 groups: 64 cases of NMF and 36 cases of MF and retrospectively compared the clinical characteristics of the 2 groups (Table 1). All patients were immunocompetent with no specific medicosurgical history. The mean age and symptom duration did not significantly differ between the 2 groups. The MF group showed male predilection (men=83.3%, women=16.7%), as compared with the NMF group (men=48.4%, women=51.6%; p=0.001). Among the various body sites, facial and leg involvement were more dominant in the NMF group than in the MF group, with statistical significance. Truncal involvement was more predominant in the MF group than in the NMF group, with statistical significance. In cases of scalp and arm involvement, no significant differences between the 2 groups were observed. Various morphologic features of folliculitis were Onoted, including papules, pustules, and papulopustules, with no significant differences between the 2 groups (p=0.589).
Folliculitis originates from follicular and perifollicular inflammation characterized by erythematous papules and pustules. Skin biopsy revealed follicular inflammatory cell infiltration with neutrophils and lymphocytes with variable findings, depending on etiology. Etiologic factors of folliculitis are numerous, such as bacterial, viral, and fungal infections, and noninfectious factors such as eosinophilic infiltration and drugs2. Among the different types of folliculitis, MF is commonly encountered by physicians. However, MF could be easily misdiagnosed as simple superficial bacterial folliculitis and treated with anti-acne medications or antibiotics rather than antifungal agents4. Because of these experiences, we decided to perform this study to determinethe likelihood of misdiagnosis of MF and analyze the relevant clinical data retrospectively.
First, we rediagnosed 10 patients who were previously diagnosed with NMF with MF on the basis of findings from serial sections stained with H&E. When dermatologists and pathologists encounter tissue slides that clinically indicate folliculitis, there is a tendency to arrive at a diagnosis of "consistent with folliculitis" or "suggestive of folliculitis" if follicular inflammation that includes neutrophils is noted. Moreover, serial section is not routinely performed unless the pathologic finding is inconclusive because the sections cannot be adequately visualized5. Through serial section of folliculitis tissues in this study, clear visualization of the hair follicles and adjacent area led to an accurate diagnosis of MF. These results indicate that a simple and easy serial section of folliculitis tissues prevents misdiagnosis of MF and prescription of improper medication.
Second, we conducted a histochemical study using the d-PAS staining method. Eosinophilic round to oval organisms are observed within follicles using this method6. Through this method, we found Malassezia in the hair follicles of 6 patients. This indicates that MF was not diagnosed by H&E staining in these 6 cases, but the causative organism was visible with d-PAS stain. Another possibility is that additional serial section for d-PAS staining enabled the detection of Malassezia. These results suggest that d-PAS stain should be considered in suspected MF cases, when yeast cells are not detected by H&E staining.
On the basis of serial section and d-PAS staining findings, we divided all the cases again into 2 groups, NMF (n=64) and MF (n=36), for comparison of clinical characteristics. The incidence of MF was 5 times higher in men than in women. This result is compatible with that of a previous report from Singapore, wherein the incidence of MF was 11 times higher in men than in women7. This sex predilection can be attributed to the metabolic and physical differences between men and women. Compared with women, men have increased physical activity, resulting in increased sweating and likelihood of infection. This sex discrepancy, however, is hard to explain precisely and requires further evaluation. The distribution pattern was quite different between the 2 groups and was thought to be remarkable. Similar to previous reports8, in our study, MF predominantly involved the trunk, whereas facial involvement and leg involvement rates were relatively low. This distributional discrepancy can be attributed to the differences in the skin environment such as sweating and chance of occlusion9,10. Some authors stated that facial involvement is quite common11. The degree of facial involvement differs depending on the patients' country of residence, subtype of Malassezia, and coexisting disease. Additional evaluations are required to clarify this controversial point. It was difficult to find differences between the 2 groups with regard to the duration and morphology of skin lesions, such as papules, pustules, and papulopustules.
This study has some limitations. We relied on data from the medical charts; some data were missing, such as pruritus, treatment outcome, and prognosis. Therefore, well-designed and prospective studies are recommended to remedy our shortcomings.
In conclusion, when physicians encounter follicular skin lesions, various diagnostic tools should be considered with Malassezia infection in mind. Especially in male patients with follicular papules or pustules located predominantly on the trunk, MF as well as other types of folliculitis should be considered in the differential diagnosis. If biopsy is performed for histologic confirmation, physicians should try to obtain numerous slides through serial section and visualize the hair follicles. In cases of patients suspected with Malassezia infection, d-PAS staining is also recommended. Through serial tissue section and d-PAS staining, the detection rate of Malassezia would improve, thereby allowing precise diagnosis and proper treatment.
Figures and Tables
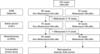 | Fig. 1Scheme of evaluation of Malassezia folliculitis (MF) and non-MF (NMF). Through serial section and histochemical study, additional Malassezia species could be visualized, and the previous diagnosis of NMF was changed to MF. H&E: hematoxylin and eosin, d-PAS: diastase-Periodic acid-Schiff. |
 | Fig. 2Through serial ttisue sections stained with hematoxylin and eosin, Malassezia are seen in the hair follicle (B, C) resulting in s change in the diagnosis from non-Malassezia folliculitis (A) to Malassezia folliculitis. |
 | Fig. 3(A) Initial slide diagnosed as non-Malassezia folliculitis. Malassezia microorganisms, not found by serial tissue section (B), but visible on diastase-Peridoc acid-Schiff (d-PAS) staining (C). |
References
1. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004; 5:301–310.
2. Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: cytology. Crit Rev Microbiol. 2013; 39:9–25.

3. Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004; 51:785–798.

4. Lévy A, Feuilhade de Chauvin M, Dubertret L, Morel P, Flageul B. Malassezia folliculitis: characteristics and therapeutic response in 26 patients. Ann Dermatol Venereol. 2007; 134:823–828.
5. Sigg C, Pelloni F, Schnyder UW. Focal melanocytic atypia in dysplastic nevus cell nevi. Results of a serial section study. Hautarzt. 1989; 40:701–707.
6. Cuadra Oyanguren J, Barbera Montesinos E, Sánchez Carazo JL, Aliaga Boniche A. Folliculitis caused by Pityrosporum. Med Cutan Ibero Lat Am. 1985; 13:357–361.
7. Lim KB, Giam YC, Tan T. The epidemiology of Malassezia (Pityrosporon) folliculitis in Singapore. Int J Dermatol. 1987; 26:438–441.

8. Budavari JM, Grayson W. Papular follicular eruptions in human immunodeficiency virus-positive patients in South Africa. Int J Dermatol. 2007; 46:706–710.

9. Bäck O, Faergemann J, Hörnqvist R. Pityrosporum folliculitis: a common disease of the young and middle-aged. J Am Acad Dermatol. 1985; 12:56–61.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download