Abstract
Neurothekeoma is a rare cutaneous neoplasm, occurring as a cutaneous papule or nodule on the face, shoulders, and upper extremities. Neurothekeoma has been subclassified as either the myxoid, cellular, or mixed type, depending on the amount of myxoid matrix and on immunohistochemical analysis. We observed a clinical case with conflicting histopathological and immunohistochemical findings. In this case, microscopic examination showed the typical presentation of myxoid neurothekeoma; however, immunohistochemical staining was negative for S100 protein and positive for CD68, which is the characteristic pattern of cellular neurothekeoma. We report a very rare form of myxoid cellular neurothekeoma of the face in a young woman.
Neurothekeoma is a rare benign dermal soft tissue tumor derived from the peripheral nerve sheath1. It usually presents as a slow-growing, solitary papule or nodule that is mainly located on the head and neck, shoulder, and upper extremities2,3,4,5. This tumor has been subclassified histopathologically into 3 groups-myxoid, mixed, and cellular type-depending on the amount of myxoid matrix2. Immuhistochemical markers such as S100 protein, glial fibrillary acidic protein (GFAP), nerve growth factor receptor (NGFR), CD57, NKI/C3, Ki-M1p, and CD68 can be applied to the tumor cells to distinguish among these 3 subtypes of neurothekeoma1,2,6,7,8,9,10,11. Up to date, only a single case of "myxoid cellular neurothekeoma" has been reported12. Myxoid cellular neurothekeoma typically presents histological features of the myxoid type but is negative for S100 protein, CD57, or GFAP and positive for NKI/C3, Ki-M1p, or CD68. Herein, we report a case of a myxoid cellular neurothekeoma with a review of related literature.
A 30-year-old woman presented with a 5-year history of a slow-growing, painless nodule on the medial side of the left eyebrow. She did not have any history of underlying diseases or trauma. On physical examination, a 0.7-×0.6-cm erythematous firm nodule was observed on the medial side of the left eyebrow (Fig. 1). An excisional biopsy was taken. Histopathologiccal examination of the excised specimen showed lobular dermal tumor nodules composed of slender spindle-shaped and stellate cells loosely interconnected within an abundant myxoid stroma (Fig. 2). The amount of myxoid matrix was found to be >50% by using alcian blue staining (Fig. 2C). Immunohistochemical staining demonstrated positivity for CD68 and vimentin, and negativity for S100 protein, CD57, GFAP, and chromogranin (Fig. 3). Although it should be histopathologically classified as a myxoid type, it also possesses characteristics of the cellular type according to immunohistochemical staining. Consequently, we confirmed the diagnosis of myxoid cellular neurothekeoma. There was no evidence of recurrent disease at a 1-year follow-up.
Neurothekeoma is a benign, cutaneous, neurogenic neoplasm that can be classified into the myxoid, cellular, and mixed types primarily on the basis of the amount of myxoid matrix. In the study by Fetsch et al.2, tumors with ≤10% myxoid matrix were classified as cellular neurothekeomas and those with >10% but ≤50% myxoid matrix were designated as mixed-type neurothekeomas. Myxoid neurothekeomas had >50% myxoid matrix. The classic or myxoid type was first described as a 'myxoma of the nerve sheath', occurring primarily in adults, with a predilection for the head and neck region in women1. Clinically, myxoid neurothekeomas are solitary asymptomatic nodules, often mistaken as dermal nevi, cysts, or adnexal neoplasms2. The histopathological findings are lobular to plexiform dermal tumor masses composed of spindle- to stellate-shaped cells, with a striking myxoid stroma13. Tumor cells are typically positive for markers of nerve-origin cells, such as S100 protein, GFAP, and NGFR. However, the cells were not stained with macrophage markers including Ki-M1p and CD68, and they were considered to be derived from nerve cells or Schwann cells1,2,4,14.
A similar but more cellular variant, with less mucin content and ill-defined growth, were the cellular neurothekeomas first described in 1986 by Rosati et al.15, and they are commonly observed in the head and neck region of young persons. Histopathologically, these dermal tumors are poorly circumscribed, typically composed of fascicles of spindle-shaped to epithelioid tumor cells, and absent or sparse mucinous matrix13. Unlike the myxoid type, the tumor cells of the cellular variant are not generally stained with markers for nerve-origin cells, such as S100 protein, GFAP, NGFR, and CD57, but are positively stained with NKI/C3, Ki-M1p, and CD68. Therefore, it is postulated that they show a fibrohistiocytic differentiation1,6,7,8,9,10,11. Mixed-type neurothekeomas show overlapping features of both variants. Immunohistochemistry analyses of these cases often show confusing features with an irregular or absent reactivity to S100 protein and smooth muscle actin13. Neuroectodermal antigens such as neuron-specific enolase, NKI/C3, PGP 9.5, or leu-7, and fibrohistiocytic antigens including factor XIIIa, vimentin, EMA, or SMA have limited diagnostic value in classifying the types of neurothekeoma. These antigens lack diagnostic specificity owing to insufficient reproducibility and inconsistent presentations depending on the literature report2,4. The S100 protein is known to be useful in differentiating the myxoid type from the cellular type5; however, Strumia et al.14 reported conflicting findings. The authors found 'S100 negative myxoid neurothekeoma' that was negatively stained with S100 protein. Up to now, there are 3 reported cases of S100-negative myxoid neurothekeoma in the English-language literature16,17.
However, in all of the 3 cases, the authors did not perform immunohistochemical staining with fibrohistiocytic markers such as CD68 or Ki-M1p, which are positive in cellular neurothekeoma.
Rudolph and Schubert12 reported a 'myxoid cellular neurothekeoma' that presented the typical histological features of the myxoid type but was negative for S100 protein or NGFR and positive for NKI/C3 and Ki-M1p. Therefore, it is important to use various immunohistochemical staining techniques instead of depending solely on histological presentation during diagnosis. Hematoxylin and eosin staining of our case revealed an abundant amount of myxoid matrix (>50% with alcian blue staining) and stellate to spindle-shaped cells loosely arranged in the matrix. Although this is a typical histological feature of myxoid neurothekeoma, immunohistochemical examination showed negative staining for S100 protein, CD57, and chromogranin and positive staining for CD68 and vimentin (Table 1). Therefore, it was diagnosed as a myxoid cellular neurothekeoma as suggested by Rudolph and Schubert12. Neurothekeoma is slow-growing benign tumor and is asymptomatic. It is treated with simple excision because no metastasis has been reported14. However, it can recur if the lesion is incompletely excised1. In this case, no recurrence was observed since a simple excision was performed 1 year ago. Up to now, myxoid cellular neurothekeoma has not been reported in Korea. This is the second case report worldwide besides the one suggested by Rudolph and Schubert12.
We conclude that in the case of an S100-negative myxoid neurothekeoma, immunohistochemical staining with fibrohistiocytic markers such as CD68 should be performed to differentiate from myxoid cellular neurothekeoma, which is a new entity.
Figures and Tables
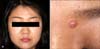 | Fig. 1(A) A solitary erythematous, 0.7-×0.6-cm, telangiectatic nodule on the left eyebrow. (B) The nodule has a smooth and shiny surface with some telangiectasia. |
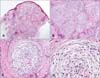 | Fig. 2Histopathological findings. (A) The tumor is in the dermis and lobulated, not encapsulated (H&E, ×20). (B~D) The tumor cells were composed of stellate and spindle-shaped cells within an abundant myxoid stroma (H&E; B: ×100, C: ×200, D: ×400). |
 | Fig. 3Immunohistochemical findings. (A) The amount of myxoid stroma was >50% (alcian blue, ×40). (B, C) The tumor cells were positive for CD68 and vimentin (B: CD68, ×100; C: vimentin, ×100). The tumor cells were negative for S100 protein (D, ×100), CD57 (E, ×100), and glial fibrillary acidic protein (F, ×100). |
References
1. Papadopoulos EJ, Cohen PR, Hebert AA. Neurothekeoma: report of a case in an infant and review of the literature. J Am Acad Dermatol. 2004; 50:129–134.

2. Fetsch JF, Laskin WB, Hallman JR, Lupton GP, Miettinen M. Neurothekeoma: an analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am J Surg Pathol. 2007; 31:1103–1114.

3. Yang YW, Shih IH, Huang YH, Kuo TT, Hong HS. Mixed-type neurothekeoma presenting with an unusual clinical appearance of multiple satellite lesions on the back. Dermatol Surg. 2005; 31:720–722.

4. Oh SH, Lee HJ, Chang SE, Lee MW, Choi JH, Moon KC, et al. A case of cellular neurothekeoma. Korean J Dermatol. 2006; 44:1126–1129.
5. Ryu DJ, Kim HJ, Jung JY, Kwon YS, Lee JH. A case of myxoid neurothekeoma on the hand. Korean J Dermatol. 2009; 47:982–985.
6. Chang SE, Lee TJ, Ro JY, Choi JH, Sung KJ, Moon KC, et al. Cellular neurothekeoma with possible neuroendocrine differentiation. J Dermatol. 1999; 26:363–367.

7. Husain S, Silvers DN, Halperin AJ, McNutt NS. Histologic spectrum of neurothekeoma and the value of immunoperoxidase staining for S-100 protein in distinguishing it from melanoma. Am J Dermatopathol. 1994; 16:496–503.

8. Mahalingam M, Alter JN, Bhawan J. Multiple cellular neurothekeomas--a case report and review on the role of immunohistochemistry as a histologic adjunct. J Cutan Pathol. 2006; 33:51–56.

10. Laskin WB, Fetsch JF, Miettinen M. The "neurothekeoma": immunohistochemical analysis distinguishes the true nerve sheath myxoma from its mimics. Hum Pathol. 2000; 31:1230–1241.

11. Argenyi ZB, LeBoit PE, Santa Cruz D, Swanson PE, Kutzner H. Nerve sheath myxoma (neurothekeoma) of the skin: light microscopic and immunohistochemical reappraisal of the cellular variant. J Cutan Pathol. 1993; 20:294–303.

13. Müller CS, Tilgen W, Kutzner H, Pföhler C. Recurring mixed-type neurothekeoma of the face. Dermatoendocrinol. 2009; 1:220–222.

14. Strumìa R, Lombardi AR, Cavazzini L. S-100 negative myxoid neurothekeoma. Am J Dermatopathol. 2001; 23:82–83.

15. Rosati LA, Fratamico FC, Eusebi V. Cellular neurothekeoma. Appl Pathol. 1986; 4:186–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download