Abstract
Lichen nitidus (LN) is an uncommon, usually asymptomatic cutaneous eruption characterized by the presence of multiple, small, flesh-colored papules. The epidemiologic and pathophysiologic characteristics of LN have not yet been defined. Furthermore, LN has rarely been described in association with other cutaneous diseases. We herein report 3 cases of LN associated with various cutaneous diseases, including lichen striatus, oral lichen planus, and psoriasis vulgaris.
Lichen nitidus (LN) is an uncommon idiopathic inflammatory cutaneous eruption first described by Pinkus in 19011. The dermatosis is composed of multiple, small, discrete, glistening, flesh-colored to slightly pink papules that may occur anywhere on the skin; however, the most common sites are the glans and shaft of the penis, genitalia, abdomen, and extremities1,2. Involvement of the palms and soles, nails, and mucous membranes has rarely been described3,4,5,6. As in lichen planus (LP), the Koebner phenomenon is observed and is the hallmark of LN1,2,7. The lesions are usually asymptomatic but may be mildly pruritic. Histopathologically, LN is characterized by a well-circumscribed, mixed-cell granulomatous infiltrate near the lower surface of the epidermis and confined to a widened dermal papillae8.
The clinical and histopathologic features of LN are quite definitive; however, the etiology and pathogenesis remain obscure1. It has been proposed that LN may be associated with immune alterations in the patient7. The relation between LN and LP has been debated by many investigators1. A few reports have demonstrated that LN is associated with other diseases, including LP9,10,11,12, erythema nodosum10, segmental vitiligo13, and lichen spinulosus14. We herein report 3 rare cases of LN associated with lichen striatus (LS), oral LP (OLP), and psoriasis vulgaris.
A 12-year-old boy presented with a 1-year history of pruritic papules on the right upper back and right arm. His medical history and family history were unremarkable, and there was no relevant history of systemic or topical drug use. Physical examination revealed well-defined, pinhead-sized, skin-colored to erythematous grouped papules on the right upper back, and pinhead-sized, whitish papules in a linear configuration on the right arm (Fig. 1A, B).
Histopathologic examination of the papules on the right upper back revealed hyperkeratosis, acanthosis, hydropic degeneration of the basal cell layer, and a circumscribed nest of inflammatory cells in 2 contiguous dermal papillae ('claw clutching a ball' appearance) (Fig. 1C). Furthermore, skin biopsy from the right arm showed hyperkeratosis, focal parakeratosis, acanthosis, hydropic degeneration of the basal cell layer, superficial perivascular lymphocytic infiltration, and perifollicular lymphohistiocytic infiltration (Fig. 1D).
The histopathologic findings were consistent with LN on the right upper back and LS on the right arm. Because the patient complained of mild itching of the lesions, he was treated with topical methylprednisolone aceponate and 0.1% topical tacrolimus for 3 months. He is currently being observed and has had no recurrence.
33-year-old woman presented with a 6-month history of slightly erythematous plaques with whitish striae arranged in a lacy pattern on the tongue, as well as relatively well-defined, skin-colored to slightly erythematous, grouped papules on both wrists (Fig. 2A, B). She did not complain of itching and pain. Her medical history and family history were unremarkable, and she was not taking prescribed medicines. Routine laboratory examinations, including peripheral blood cell analysis, liver function tests, and renal function tests, were all within normal limits.
A skin biopsy from the tongue revealed hyperkeratosis, acanthosis, vacuolar alteration of the basal layer, and band-like infiltration in the papillary dermis (Fig. 2C). The biopsy specimen from the left wrist showed parakeratosis and a claw clutching a ball appearance. On the basis of the clinical course and histopathological characteristics, the lesions on the patient's tongue were diagnosed as OLP, whereas the lesions on both wrists were diagnosed as LN.
The patient was treated with 4 mg/day (0.1 mg·kg-1·day-1) oral methylprednisolone, topical methylprednisolone aceponate, and 0.1% topical tacrolimus. The treatment was continued for 3 months but none of the lesions were improved; therefore, 100 mg/day (2.5 mg·kg-1·day-1) cyclosporine was administered. She did not complain of any adverse effects of drugs. After 1 year of oral cyclosporine therapy, the OLP and LN lesions began to improve nearly simultaneously. She is currently under observation without recurrence.
An 8-year-old boy presented with 2 distinct types of cutaneous lesions. He presented with a 6-month history of scattered, relatively well-defined, pea- to bean-sized, erythematous, scaly plaques on the trunk, extremities, and face (Fig. 3A). Additionally, he had scattered, well-defined, pinhead-sized, skin-colored, glistening papules on the trunk for 6 months (Fig. 3B). He did not complain of itching and pain. He had no significant medical or family history. Results of routine laboratory examinations, including peripheral blood cell analysis, liver function tests, and renal function tests, were all within normal limits.
Skin biopsy from the erythematous scaly plaques on the right knee revealed spongiform pustules of Kogoj and parakeratotic mounds (Munro microabscesses), hyperkeratosis, diminished granular layer, acanthosis with elongation of the rete ridges, and dilated capillaries at the superficial dermis with perivascular lymphocytic infiltration (Fig. 3C). These histological findings suggested psoriasis vulgaris. Skin biopsy from the glistening papules on the trunk showed parakeratosis, vacuolar alteration of the basal cell layer, and a claw clutching a ball appearance. These findings suggested LN.
After the diagnosis, narrow-band ultraviolet B (NB-UVB) phototherapy twice a week was initiated at 200 mJ/cm2, along with the application of topical methylprednisolone aceponate and topical acitretin ointment. The skin lesions of LN were improved after 14 irradiation courses with a cumulative dose of 12.5 J/cm2, and were almost cured with a cumulative dose of 31 J/cm2. After the NB-UVB phototherapy, the skin lesions of psoriasis remained reduced in size and number. He is currently being observed with continued application of topical steroid and acitretin ointment.
LN is an uncommon cutaneous eruption with well-defined clinical and histopathologic features. The majority of LN cases occur in children and young adults1. The dermatosis is characterized by multiple, small, flesh-colored papules commonly found on the genitalia, abdomen, and extremities. Rarely, the mucous membranes, nails, palms, and soles may be involved. Additionally, vesicular, hemorrhagic, perforating, spinous follicular, linear, generalized, and actinic variants have also been reported2. The histological hallmark of the disease is a well-circumscribed mixed-cell (including lymphocytes and multinucleated epithelioid histiocytes) granulomatous infiltrate in the papillary dermis, embraced by elongated rete ridges, suggesting a claw clutching a ball configuration8.
The clinical and histopathologic features of LN are quite characteristic; however, the etiology and pathogenesis are still poorly understood. It has been proposed that LN may be associated with immune alterations in the patient7. Some authors have argued that LN represents a variant of LP, and the coexistence of the 2 diseases has been reported in some patients9,10,11,12. Other authors have stated that these diseases are distinct entities, on the basis of histological differences. On the basis of immunohistochemical analyses, Smoller and Flynn15 have revealed that LN indeed is not a localized papular variant of LP. They found that the T-cell infiltrate in LN was more heterogeneous (characterized by a pleomorphic mixture of helper and nonhelper T cells and macrophages) than that in LP, which is characterized by a predominance of helper T cells. Nevertheless, similar histologic findings in early lesions of both diseases have been reported12. Therefore, the close association between LN and LP suggests that these 2 diseases may possibly be different manifestations of similar etiologic factors12.
Another etiologic theory of LN proposes that an active infectious agent or idiopathic response to nonviable microbial antigens (viruses, mycobacterial and treponemal species, streptococci) may activate a cell-mediated response, initiate lymphocyte accumulation, and form inflammatory papules1,16. Berger and Dhar17 reported that LN lesions developed as a lichenoid photoeruption in advanced human immunodeficiency virus-infected patients. Moreover, a case of generalized LN in a patient with hepatitis C after systemic treatment with interferon α and ribavirin has been reported16. A relation between immunological alterations and the development of LN has also been suggested.
LN and LS are distinct entities, and the relation between these diseases has been largely neglected. However, there are several case reports of showing typical histologic features of LN (a claw clutching a ball configuration of the epidermis) and histologic features of LS (periappendageal infiltration) simultaneously18,19. Furthermore, there are cases of LN presenting in a linear distribution, clinically mimicking LS20. Sanders et al.18 suggest a morphologic spectrum between LN and LS. In our cases, we observed the patient who developed both LN and LS that showed clinically and histologically distinct features. Our case should encourage further investigations of the relation between LN and LS.
To the best of our knowledge, there has been no reported cases of coexisting psoriasis vulgaris and LN in the Korean and foreign literature. In our third case, the 2 diseases could be present in the same patient by accident. However, further data are necessary to discern whether the coexistence of LN and psoriasis vulgaris is an isolated coincidence, or if there is a causative link between the 2 diseases.
LN has been rarely described in association with other diseases, including LP9,10,11,12, erythema nodosum10, segmental vitiligo13, and lichen spinulosus14. The basic clinical reports of LN associated with other cutaneous diseases are summarized in Table 1.
In conclusion, we have described 3 cases of LN associated with various cutaneous diseases, including LS, OLP, and psoriasis vulgaris. We could not prove the interrelation between LN and these diseases, and reported only 3 cases. However, we suggest that various cutaneous diseases concurred with LN. Extensive studies are required to determine whether LN is independent of these other cutaneous diseases or if they have a common pathogenesis.
Figures and Tables
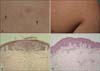 | Fig. 1Physical examination of the patient 1 revealed well-defined, pinhead-sized, skin-colored to erythematous grouped papules (arrows) on the right upper back (A), and pinhead-sized, whitish papules in a linear configuration on the right arm (B). Histopathologic examination from the right upper back shows hyperkeratosis, acanthosis, hydropic degeneration of the basal cell layer, and 'claw clutching a ball' appearance (C), from the the right arm shows hyperkeratosis, focal parakeratosis, acanthosis, hydropic degeneration of the basal cell layer, superficial perivascular lymphocytic infiltration and perifollicular lymphohistiocytic infiltration (D) (C, D: H&E, ×100). |
 | Fig. 2Physical examination of the patient 2 revealed slightly erythematous plaques with whitish striae arranged lacy pattern on the dorsal surface on the tongue (A), and localized, relatively well defined, 2 to 3 mm sized, skin colored to slightly erythematous, grouped papules on both wrists (B). (C) Biopsy from the tongue shows hyperkeratosis, hypergranulosis, irregular acanthosis, hydropic degeneration of basal cells and band like infiltration of lymphocytes in the upper dermis (H&E, ×100). |
 | Fig. 3Physical examination of the patient 3 revealed scattered, relatively well-defined, erythematous, scaly plaques on the lower extremities (A), and scattered, well-defined, pinhead-sized, skin colored, glistening papules on the trunk (B). (C) Biopsy from the right knee shows parakeratotic mounds (Munro microabscesses), hyperkeratosis, acanthosis with elongation of rete ridges and dilated capillaries at the superficial dermis with perivascular lymphocytic infiltration (H&E, ×100). |
References
1. Pinkus F. Verhand. Berlin Dermat Gesel. 1901; 12:3.
2. Park JH, Kye YC, Kim SN. A clinical and histopathologic study of lichen nitidus. Korean J Dermatol. 2003; 41:857–868.
3. Munro CS, Cox NH, Marks JM, Natarajan S. Lichen nitidus presenting as palmoplantar hyperkeratosis and nail dystrophy. Clin Exp Dermatol. 1993; 18:381–383.

4. Thibaudeau A, Maillard H, Croué A, Belperron P, Avenel Audran M, Verret JL. Palmoplantar lichen nitidus: a rare cause of palmoplantar hyperkeratosis. Ann Dermatol Venereol. 2004; 131:822–824.
5. Natarajan S, Dick DC. Lichen nitidus associated with nail changes. Int J Dermatol. 1986; 25:461–462.

6. Krook G. Purpura in lichen nitidus generalisatus. Report of a case. Acta Derm Venereol. 1959; 39:238–246.
7. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004; 51:606–624.

8. Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott-Raven;2009. p. 192–193.
9. Aram H. Association of lichen planus and lichen nitidus. Treatment with etretinate. Int J Dermatol. 1988; 27:117.
10. Kano Y, Shiohara T, Yagita A, Nagashima M. Erythema nodosum, lichen planus and lichen nitidus in Crohn's disease: report of a case and analysis of T cell receptor V gene expression in the cutaneous and intestinal lesions. Dermatology. 1995; 190:59–63.

11. Kawakami T, Soma Y. Generalized lichen nitidus appearing subsequent to lichen planus. J Dermatol. 1995; 22:434–437.

12. Di Lernia V, Piana S, Ricci C. Lichen planus appearing subsequent to generalized lichen nitidus in a child. Pediatr Dermatol. 2007; 24:453–455.

13. Agarwal S, Guglani V, Kumar B. Down's syndrome with lichen nitidus and segmental vitiligo. Indian J Dermatol Venereol Leprol. 2009; 75:627–629.

14. MacDonald AJ, Drummond A, Chui D, Holmes S. Lichen nitidus and lichen spinulosus or spinous follicular lichen nitidus? Clin Exp Dermatol. 2005; 30:452–453.

15. Smoller BR, Flynn TC. Immunohistochemical examination of lichen nitidus suggests that it is not a localized papular variant of lichen planus. J Am Acad Dermatol. 1992; 27:232–236.

16. Scheler M, Proelss J, Bräuninger W, Bieber T, Wenzel J. Generalized lichen nitidus with involvement of the palms following interferon alpha treatment. Dermatology. 2007; 215:236–239.

17. Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994; 130:609–613.

18. Sanders S, Collier DA, Scott R, Wu H, MeNutt NS. Periappendageal lichen nitidus: report of a case. J Cutan Pathol. 2002; 29:125–128.

19. Madhok R, Winkelmann RK. Spinous, follicular lichen nitidus associated with perifollicular granulomas. J Cutan Pathol. 1988; 15:245–248.

20. Petrozzi JW, Shmunes E. Linear lichen nitidus. Cutis. 1970; 6:1109–1112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download