Abstract
Intravascular lymphoma (IVL) is a rare disorder characterized by the presence of large neoplastic lymphoid cells restricted to the lumens of small vessels with a predilection for the skin and the central nervous system. While the vast majority of cases involving IVL are of B-cell lineage, the disease rarely affects the T-cell, the histiocytes, and the natural killer cells. We report a case of intravascular T-cell lymphoma (IVTL) associated with Epstein-Barr virus (EBV). A 23-year-old healthy woman presented with tender indurated erythematous patches with overlying telangiectasia on her right breast, abdomen, both the upper and the lower extremities and the back for 3 months. The pathology revealed an infiltration of dermal and subcutaneous vessels by large and atypical lymphoid cells with immunohistochemical features of the T-cell lineage with a cytotoxic phenotype (CD3+, CD8+, granzyme B+, TIA-1+, CD4-, CD5-, CD20-, CD56-). Interestingly, the DNA extracted from the skin biopsies demonstrated evidence of a monoclonal immunoglobulin heavy chain gene rearrangement, but no T-cell receptor gene rearrangement was found. In situ hybridization study for EBV-encoded RNA was positive. She was diagnosed with an EBV-associated IVTL. The patient's skin lesions were refractory to the combination of chemotherapy and autologous stem cell transplant, and she expired. The findings in the present case may highlight the unique clinicopathologic aspects of EBV-associated cytotoxic IVTL that occurred in a young, immunocompetent woman.
Intravascular lymphoma (IVL) is a rare sub-subtype of a common subtype of non-Hodgkin's lymphoma, an extranodal diffuse large B-cell lymphoma. This disorder was first described as angioendotheliomatosis proliferans systemisata by Pfleger and Tappeiner1 in 1959. The neoplastic cells were initially believed to be of endothelial origin. Subsequent immunohistochemical and molecular studies demonstrated the lymphoid nature of the neoplastic cells. Because IVL is an extranodal disease, this means that symptoms will vary, depending on the extranodal organ that is being affected2. Generalized symptoms may include fatigue, but perhaps the key clinical feature of IVL is vascular obstruction and the resulting edema depending on the affected organs. The extranodal sites most frequently involved in IVL are the skin and the central nervous system (CNS), but it has also been known to affect the kidney, the lung, and the gastrointestinal tract, among other organs2. Unlike in other lymphomas, lymph nodes and bone marrow are rarely affected. Most IVLs have a B-cell phenotype, but the T-cell and the natural killer (NK)-cell variants have occasionally been reported3. In a series of 38 cases of IVL, only 1 (2.6%) had a T-cell phenotype4. We describe a typical case of Epstein-Barr virus (EBV)-associated intravascular T-cell lymphoma (IVTL) in a young, immunocompetent woman.
A 23-year-old healthy woman presented with ill-defined indurated dusky erythematous patches with fine red telangiectasias on her right breast, abdomen, both the upper and the lower extremities and the back for 3 months (Fig. 1). The skin lesions were slightly tender and they had gradually increased in size. Initially, the patient's skin lesions were clinically diagnosed as simple red telangiectasia at another university hospital and she had several treatment sessions of vascular laser on her skin lesions with no improvement. The skin biopsy specimen from the right breast demonstrated large atypical hematopoietic cells strictly confined to the lumens of the vessels of the mid to deep dermis and the subcutaneous tissue. These atypical cells have large irregular hyperchromatic nuclei, prominent nucleoli, and a moderate amount of cytoplasm. Abnormal mitosis was prominent in the neoplastic cells and a number of red blood cells were admixed with the neoplastic cells (Fig. 2). On the immunohistochemical studies, these cells were positively stained with CD3, CD8, granzyme B, TIA-1, myeloperoxidase, leukocyte common antigen and Ki-67 (50%~100%) (Fig. 3A~E). However, they were negatively stained with CD4, CD5, CD20, CD30, CD56, and cytokeratin. In situ hybridization study for EBV-encoded RNA (EBER) was positive (Fig. 3F). Through molecular study, the immunoglobulin heavy chain (IgH) gene rearrangement was defined as monoclonality, whereas the T-cell receptor (TcR)-γ gene rearrangement was proven as polyclonality. A bone marrow biopsy resulted in normocellular marrow (70%) with no atypical cells and the cerebrospinal fluid examination did not reveal any atypical cells. Furthermore, a hematologic work-up, including a complete blood count, a computed tomography scans of the chest, abdomen, and the pelvis, and positron emission tomography scan, was negative. She was diagnosed as IVTL. She was treated with cyclophosphamide, doxorubicin, vincristine and predisolone in addition to the intrathecal methotrexate for 6 cycles. After the chemotherapy, only a faint erythema was checked on her right breast, and postinflammatory hyperpigmentation remained on the extremities. Moreover, no skin lesion was observed on her back. A follow-up skin biopsy from her right breast demonstrated no tumor cell, and she was received an allo-peripheral blood stem cell transplantation (PBSCT). But, after 4 months from the allo-PBSCT, ill-defined walnut sized erythematous patches developed on her right breast, which was diagnosed as a recurrence of IVTL through biopsy. She received a second chemotherapy at another hospital, but the response was poor. She expired due to a neutopenic fever with pneumonia 11 months after the recurrence.
IVL is an unusual subtype of an extranodal large cell lymphoma, a generally systemic disease with a predilection for the skin and the CNS involvement. Histopathologically, it was characterized by the presence of tumor cells in the lumina of small vessels5. Despite the intravascular localization of the disease, circulating neoplastic cells are rarely identified in the peripheral blood6. Affecting patients in their middle-age and older, IVL, as an extranodal disease, can involve virtually any organ in the body. Approximately two-thirds of the patients are presented with cutaneous or neurologic symptoms. The cutaneous manifestations are varied and include painful indurated erythematous eruptions, ill-defined violaceous plaques, erythematous and desquamative plaques, cellulitis-like lesions, erythematous papules, painful red-blue nodules, and ulcerated nodules6. Neurologic findings may also occur, including dementia, polyneuropathy, myalgia, and muscle weakness. Other clinical findings include fever, anorexia, weight loss, malaise, and signs and symptoms reflecting the microvascular occlusions at various sites7. IVL usually follows an aggressive clinical course, with median survival times ranging from 5 to 13 months.
A review of reported cases of IVL revealed that approximately 85% to 90% are of a B-cell lineage and 10% to 15% are of a T-cell origin, although the incidence of IVTL cases reported in the literature might be overestimated7. Owing to the rarity of the IVTL cases, they are more likely to be reported. Takahashi et al.8, have reviewed details for the 25 cases of IVTL documented in the English literature; one stillborn, one renal allograft recipient, and two human immunodeficiency virus (HIV)-positive cases with acquired immunodeficiency syndrome are included. Remarkably, the latter three cases among the reported 25 cases were in their third decade of life as well as the present case. The remaining 21 were mainly elderly with a mean age of 58 years, a median age of 61 years, and with a male predominance (male-to-female ratio of 13 : 7). Most cases of IVTL have been presented with a neurologic or cutaneous involvement or a fever of unknown origin. Other presentations include an interstitial lung disease9 and the infiltration of a testis10. Postmortem studies have shown that most cases have a multiorgan involvement. A renal involvement is particularly common, suggesting that a renal biopsy may be both a means of diagnosis and to follow response to the treatment11. However, our patient was young and immunocompetent. Furthermore, she has had an indolent course with an apparently localized cutaneous disease, 24 months after the onset of the symptoms.
In an intravascular NK/T-cell lymphoma, the molecular analysis of TcR gene rearrangement by polymerase chain reaction (PCR) was conducted in only a few instances, revealing a monoclonality in approximately one-third of the cases12, with the he negative cases possibly representing those with a genuine NK cell phenotype. The present case showed a negative NK cell phenotype, but polyclonality in the TcR-γ gene rearrangement. A PCR analysis of the TcR gene rearrangements, however, does not detect approximately 20% of the T-cell clones13. Furthermore, it is well documented that approximately 10% of the T-cell lymphomas have a clonal IgH gene rearrangement, which is of a similar degree of magnitude as the detection of the TcR-γ rearrangement in B-cell lymphomas (approximately 5% of cases)14. Therefore, despite the clonal IgH gene rearrangement, the present case was diagnosed as IVTL based on H&E, the immunohistochemical findings, and the results of in situ hybridization for EBV.
The near-constant association of EBV with nasal NK/T cell lymphomas suggests a probable pathogenetic role for the virus, and some authors have suggested that EBV may play a role in IVTL as well. EBV is generally absent in the IVL of B-cell origin, having been reported in only 3 of the 39 cases, two of which occurred in immunosuppressed patients. In contrast, EBV was detected in 3 of the 7 IVTL cases associated with EBV infection analyzed to date6 and also in this case. Therefore, EBV might play a role in the pathogenesis of IVTL. However, further studies are needed to establish an etiologic role for the virus.
The molecular basis for the distinctive intravascular growth pattern of IVL remains elusive. Although defects in the homing receptors CD11a/CD18 have long been considered a cause of the tumor cells' inability to extravasate, the presence of both CD11a and CD18 in the cases of IVTL has been demonstrated. Thus, CD11a/CD18-mediated mechanisms cannot fully explain the intravascular location of the tumor cells15. Another study demonstrated that CD8-positive T cells specific to HIV and EBV lacked the homing receptors CCR7 and the L-selectin (CD62L) necessary for the lymphocytes to cross the high endothelial venule barriers to enter the lymph nodes, excluding them from the immune surveillance and the antiviral defense mechanisms. It is conceivable that this lack of homing receptor could also be responsible for the intravascular localization of the lymphoma cells16.
In conclusion, we have reported a rare case of EBV-associated cytotoxic IVTL in a young, immunocompetent patient. The present case is unique since IVTL is very rare in a young, immunocompetent individual which makes the present case worthy of a report. To our knowledge, this is the first report on the cutaneous EBV-associated IVTL in the Korean dermatologic literature. IVTL is an aggressive disease that is difficult to diagnose due to its multifaceted clinical presentations. Homogeneous studies on a larger number of patients, as well as the re-evaluation of the cases published with incomplete phenotypic data would be necessary to get more information on this rare type of lymphoma.
Figures and Tables
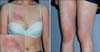 | Fig. 1A 23-year-old Korean woman with increasing number of erythematous indurated tender patches with telangiectasia over right breast, abdomen, both upper and lower extremities and back for 3 months (inset: close-up view of skin lesion in the right breast). |
References
1. Pfleger L, Tappeiner J. On the recognition of systematized endotheliomatosis of the cutaneous blood vessels (reticuloendotheliosis?). Hautarzt. 1959; 10:359–363.
2. Cerroni L, Massone C, Kutzner H, Mentzel T, Umbert P, Kerl H. Intravascular large T-cell or NK-cell lymphoma: a rare variant of intravascular large cell lymphoma with frequent cytotoxic phenotype and association with Epstein-Barr virus infection. Am J Surg Pathol. 2008; 32:891–898.
3. Rieger KE, Polidore T, Warnke R, Kim J. ALK-negative systemic intravascular anaplastic large cell lymphoma presenting in the skin. J Cutan Pathol. 2011; 38:216–220.

4. Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, International Extranodal Lymphoma Study Group (IELSG), et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the 'cutaneous variant'. Br J Haematol. 2004; 127:173–183.

5. Wang L, Li C, Gao T. Cutaneous intravascular anaplastic large cell lymphoma. J Cutan Pathol. 2011; 38:221–226.

6. Gleason BC, Brinster NK, Granter SR, Pinkus GS, Lindeman NI, Miller DM. Intravascular cytotoxic T-cell lymphoma: a case report and review of the literature. J Am Acad Dermatol. 2008; 58:290–294.

7. Wu H, Said JW, Ames ED, Chen C, McWhorter V, Chen P, et al. First reported cases of intravascular large cell lymphoma of the NK cell type: clinical, histologic, immunophenotypic, and molecular features. Am J Clin Pathol. 2005; 123:603–611.

8. Takahashi E, Kajimoto K, Fukatsu T, Yoshida M, Eimoto T, Nakamura S. Intravascular large T-cell lymphoma: a case report of CD30-positive and ALK-negative anaplastic type with cytotoxic molecule expression. Virchows Arch. 2005; 447:1000–1006.

9. Suh CH, Kim SK, Shin DH, Chung KY, Kim SK. Intravascular lymphomatosis of the T cell type presenting as interstitial lung disease--a case report. J Korean Med Sci. 1997; 12:457–460.

10. Au WY, Shek WH, Nicholls J, Tse KM, Todd D, Kwong YL. T-cell intravascular lymphomatosis (angiotropic large cell lymphoma): association with Epstein-Barr viral infection. Histopathology. 1997; 31:563–567.

11. Williams G, Foyle A, White D, Greer W, Burrell S, Couban S. Intravascular T-cell lymphoma with bowel involvement: case report and literature review. Am J Hematol. 2005; 78:207–211.

12. Cerroni L, Gatter K, Helmut K. Skin lymphoma: the illustrated guide. 3th ed. Philadelphia: WB Sauders;2009. p. 178–179.
13. Cossman J, Zehnbauer B, Garrett CT, Smith LJ, Williams M, Jaffe ES, et al. Gene rearrangements in the diagnosis of lymphoma/leukemia. Guidelines for use based on a multiinstitutional study. Am J Clin Pathol. 1991; 95:347–354.

14. Thériault C, Galoin S, Valmary S, Selves J, Lamant L, Roda D, et al. PCR analysis of immunoglobulin heavy chain (IgH) and TcR-gamma chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol. 2000; 13:1269–1279.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download