Abstract
Background
Recent advances in hyaluronic acid (HA) fillers and radiofrequency (RF) devices have been made in the context of skin rejuvenation and cosmetic surgery. Moreover, combination regimens with both techniques are currently being developed.
Objective
The present study was designed to examine the clinical and histologic effects of a new needle that incorporates an RF device for HA injections.
Methods
A new intradermal needle RF device (INNOfill; Pacific Pharma, Korea) was assessed in the present study. In the animal arm, procollagen production was measured by using enzyme-linked immunosorbent assay, the filler volume was quantified by incorporating a dye with filler, and the filler distribution was assessed through the changes in tissue structure. In the human arm, the efficacy of the combination regimen was assessed by using the wrinkle severity rating scale (WSRS).
Results
In the animal study, RF treatment increased procollagen production in a time-dependent fashion. The total volume was significantly increased with the RF treatment when compared with the filler injections alone, and lasted for up to 7 weeks after treatment. Additionally, the filler distribution was reduced in animals treated with RF when compared with the untreated group. In the human study, the nasolabial folds of subjects treated with RF before filler injections exhibited a significantly greater change in the WSRS score from baseline when compared with the nasolabial folds treated with filler injections alone.
Cutaneous aging is a net process resulting from intrinsic and extrinsic factors and is associated with many pathologic changes, including net reduction in dermal components, tissue degeneration, and reduced skin elasticity, all of which ultimately lead to macroscopic sagging and wrinkling. During the last decade, a number of injectable dermal fillers and laser modalities have been developed to counter the aging process.
Dermal fillers and radiofrequency (RF) devices represent two separate modalities for skin rejuvenation. Fillers were initially developed to restore diminished skin volume and are now commonly used to treat wrinkles. Whereas many different types of filler material are now available, hyaluronic acid (HA) filler is the most widely used1, given its track record of good outcomes, ease of administration, and few adverse effects. However, HA has a relatively short duration of action and necessitates frequent reinjections to maintain the desired outcomes2. RF therapy delivers uniform heat to the dermis, producing collagen remodeling and skin tightening; however, it has limited capacity to restore lost volume.
To address the limitations of both modalities, patients were treated with an RF device immediately before HA filler injections. Specifically, the device used in the current study delivers RF through an intradermal needle, thus creating tunnel-like coagulation canals inside the dermis and hypodermis. These canals theoretically act as protective barriers against external oxygen radicals, while simultaneously containing the injected HA filler and restricting filler spread. This combined treatment may prolong the duration of HA treatments and reduce the amount of HA filler required for maintenance. Furthermore, treatment with the RF device may also reduce wrinkles by inducing dermal remodeling and tightening.
In the present study, we tested the efficacy and safety of this new device in both animal and human clinical trials. Our data show that internal RF treatment before HA filler injections is a safe combination therapeutic regimen that synergistically improves skin folds and wrinkling.
For this study, a minimally invasive bipolar RF device (INNOfill; Pacific Pharma, Seoul, Korea; 1~2 MHz, power range 0~23 W) with 21-, 27-, or 30-gauge RF needle electrodes in combination with a patch-type electrode was evaluated (Fig. 1A). The entirety of the RF needle was insulated with a biocompatible parylene layer except the distal 1 mm, which remained exposed to act as the electrode in the tissue. A separate RF needle, specifically designed to deliver RF, was also injected into the skin to create an autologous containment collagen canal to extend the in vivo half-life of the filler (Fig. 1). The procedural protocol for the INNOfill is briefly summarized in Fig. 1B.
Yucatan pig's porcine skin and intact subcutaneous tissue were harvested by using a 5×10 cm piece of the sample. The entire sample was then completely submerged in a CO2 incubator and allowed to equilibrate to 25℃. A patch-type electrode was attached to one side of the submerged porcine skin sample, and a 27-gauge RF needle electrode was inserted at 3 mm below the skin surface. The temperature change after RF treatment was measured with FLIR T400 (FLIR System Inc., Wilsonville, OR, USA).
Dermal fibroblasts were obtained from adult foreskin explants harvested from a 65-year-old male donor. All fibroblasts were cultured and passaged in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies, Paisley, UK) with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin. All cells were incubated in 5% CO2 at 37℃.
Primary cultures (passages 4~6) were grown as three-dimensional fibroblast-populated collagen lattices (FPCLs). Specifically, collagen lattices were prepared by mixing cells with a neutralized solution of collagen type I (eight parts collagen type I, 2.9 mg/ml [Sigma-Aldrich, St. Louis, MO, USA]+one part 10× DMEM), so that the final collagen and cell concentrations were 2.0 mg/ml and 1×105 cells/ml, respectively. The cell-collagen mixture was then aliquoted into 24-well culture dishes (0.5 ml/well). Once polymerized (1 h, 37℃), DMEM+10% FBS was added on top of the FPCLs in each well. After incubation for 2 days, the attached FPCLs were mechanically released from the sides of the culture plates. To induce FPCL contraction, six sites on the FPCL were then treated with bipolar RF (1 MHz, 23 W) (Fig. 2A, B). Digital images of the contracting FPCL were captured at various time points (24 h, 48 h) during the next 2 days (Fig. 2C). Contraction of lattices was determined by averaging the longest and shortest diameter of each lattice measured with calipers. The diameters were calculated and represented as the mean and standard deviation of triplicate lattices (Fig. 2D, E).
Collagen production from the fibroblasts grown within the gels was measured in the culture medium. At 2 days after RF treatment, the medium from the contracted FPCLs was collected so that the procollagen type I protein levels could be quantified by using enzyme-linked immunosorbent assay (Takara Bio, Otsu, Japan) and as per the manufacturer's protocol. Measurements of procollagen production were assessed in triplicate.
Cytotoxicity tests on FPCLs were carried out by using a WST-1 commercially available cell proliferation reagent. The assay is based on cleavage of the tetrazolium salt WST-1 by active mitochondria to produce a soluble colored formazan salt. As the conversion is performed only by viable cells, it directly correlates with the cell number. At 2 days after RF treatment, the growth medium on the contracted FPCLs was removed and replaced with 0.3 ml test solution (DMEM). Then, 30 µl of the cell proliferation reagent WST-1 was added to each well. The FPCLs were incubated for 2 hours at 37℃ in a humidified atmosphere with 5% CO2, then the microplate was thoroughly shaken for 1 minutes and the absorbance was measured at 450 nm by using a microtiter reader (model 550; Bio-Rad Laboratories, Hercules, CA, USA). The background absorbance was measured on wells containing only the dye solution. The results were expressed as percent optical density of RF treated vs. control untreated, serum-containing cultures.
The experimental protocol described below was conducted in accordance with all guidelines established by the Korean Institutional Animal Care and Use Committee. Male New Zealand white rabbits (n=3) with an approximate initial weight of 2 kg were obtained from the Hallym Experimental Animal Center in Hwaseong, South Korea. Intramuscular xylazine hydrochloride (4 mg/kg) was administered to all animals during shaving, injection, and/or RF treatments.
The study sample was divided into four treatment groups: filler, filler after 30 seconds of RF treatment, filler after 60 seconds of RF treatment, and filler after 30 seconds of needle tunneling without RF.
All RF treatments were performed by using the INNOfill device (Pacific Pharma) at 23 W (level 9 at 1 MHz) with an insulation-coated 27-gauge needle. The needle was inserted along the back of the rabbits, and RF was administered to all treatment areas while the needle was rotated 360 degrees. This procedure was repeated 10 to 20 times for 30 to 60 seconds.
After delivering the RF energy, prefilled 1-ml syringes of filler were connected to the end of the needle electrode. Next, a 0.1 ml mixture of filler (HA 23 mg/ml, Glytone 3; Pierre Fabre, Castres, France) and dye (fast green FCF; Sigma-Aldrich) was intradermally injected into the shaved backs of the animals.
At 7 weeks, the rabbits were killed to assess the changes in the tissue structure secondary to the filler and/or RF treatments. Specifically, the tissue was stained with hematoxylin-eosin (H&E), Masson's trichrome (collagen), and Verhoeff-Van Gieson (elastic fibers) to assess the presence of any remaining filler, the overall collagen response, and the presence of elastic fibers in the dermis.
Three healthy Korean male volunteers were enrolled in this study, all of whom were assessed to have nasolabial wrinkles rated as 2 (mild) or 3 (moderate) on the wrinkle severity rating scale (WSRS). The mean age of the subjects was 41.4±4.6 years (range, 35~49 years). All subjects were treated with RF on the right nasolabial fold before the filler injection (Glytone 3, Pierre Fabre), whereas the left side was treated with filler alone. A 1-ml syringe with a sterilized 27-gauge, 0.5-in needle was used for the HA filler (23 mg/ml), and the total injected volume (0.5 ml) remained the same for each nasolabial fold.
All RF treatments were administered with the INNOfill device (Pacific Pharma) at 18 W (level 7 at 1 MHz) with an insulation-coated 27-gauge needle. Specifically, the needle was inserted along the nasolabial folds, and RF was applied to all treatment areas while the needle was rotated 360 degrees. This procedure was repeated a total of four times. After delivering RF energy, the prefilled 1-ml syringe was connected to the end of the needle electrode so that the HA filler could be injected through the linear threading technique.
Subjects were assessed at 0 (baseline), 1, 4, 8, and 12 weeks after the initial treatment, and photographs were obtained at each visit. To assess the efficacy, a blinded investigator evaluated the nasolabial folds by using the WSRS (Table 1)3. Additionally, a DermaScan C 20-MHz ultrasound device (SCANNER; Cortex Technology, Hadsund, Denmark) was used at 12 weeks to evaluate any skin changes secondary to the RF and/or filler treatments. Statistical analyses were performed using PASW Statistics version 18.0 for Windows (IBM Co., Armonk, NY, USA).
The thermal profiles of the porcine skin layers are shown in Fig. 3A both in cross-section and in the plane of the needle-electrode. The thermal profile was confined within the layer of the dermis and the needle (from the proximal end to the distal aspect of the exposed metallic portion). A maximum temperature of 24℃ was observed in the epidermis at the interface between the epidermis and dermis before RF administration (Fig. 3B). After an RF pulse duration of 10 seconds, the target temperature increased by 10℃, reaching 32℃ (Fig. 3C).
The RF application algorithm for the FPCLs is shown in Fig. 2A. Significant differences were observed in FPCL contraction after 30 minutes of RF application (Fig. 2C, D). Specifically, the RF-treated FPCLs were reduced in size by approximately 38% after 2 days (Fig. 2E). De novo collagen production was also measured in the FPCL-cultured medium. RF application to FPCLs increased the procollagen production in a time-dependent manner, with significant differences in procollagen production occurring after 30 minutes of treatment with RF. The RF-treated FPCLs decreased by 37% in area after 2 days (Fig. 2F, G).
The filler depth and volume were evaluated by injecting filler and dye into the backs of the experimental rabbits. The skin at the injection site was observed to be dark in animals treated with filler alone, whereas the skin color at the injection site was much subtler in animals randomized to the RF and/or tunneling treatment groups. These results suggest that filler injected after RF treatment and/or tunneling extends deeper into the dermis. Additionally, the total volume was observed to be greater and lasted longer (7 weeks) in the RF treatment group than in animals treated with filler alone. Specifically, the experimental groups ranked by total volume were as follows: filler after RF treatment for 60 seconds, filler after RF treatment for 30 seconds, filler after tunneling for 30 seconds without RF, and filler alone (Fig. 4A).
All tissue specimens were stained with H&E to evaluate the pattern of filler distribution within the dermis ('*' sign). The filler-injected areas showed small filler particles spread in the dermis, and areas injected with filler after RF or with tunneling showed linear filler distribution in the mid or lower dermis, respectively. The distribution of filler was reduced with RF treatment when compared with that with filler alone (Fig. 4B).
The tissue specimens were also stained with Masson's trichrome to identify the extracellular matrix components, in particular the dermal collagen. A significantly greater increase in collagen bundles was observed in subjects treated with both filler and RF when compared with those treated with filler alone. Additionally, fibroplasia was also observed in larger quantities at the periphery and area around the filler after RF treatment when compared with filler injections alone.
The tissue specimens were also stained with Verhoeff-Van Gieson to assess the quantity of elastin fibers in the dermis. Classically, long, thin elastin fibers are distributed throughout the papillary and upper reticular dermis. In the results presented here, a change in elastin fiber quality was observed after treatment with RF, with the elastin fibers in the papillary and reticular dermis noted to be markedly shorter ('arrow' sign).
Concerning the change in WSRS scores at all post-baseline time points, subjects pretreated with RF achieved better outcomes than those treated with filler injections alone (Fig. 5A), as shown by serial nasolabial fold photographs taken before and after treatment (Fig. 5B). In untreated facial skin, the outer epidermal surface of the face produced a bright reflection with an associated echolucent band. A homogenous echo-poor region was also observed below this band, representing the dermis and subcutaneous tissues. At 12 weeks after treatment, the areas treated with filler injections were significantly brighter than the untreated skin. Furthermore, areas pretreated with RF before the filler injections were significantly brighter than areas treated with the filler injections alone, suggesting that collagen synthesis may be upregulated by RF in human skin (Fig. 5C). The experimental procedure was well tolerated by all participants, none of whom reported any serious adverse events.
As interest in aging and rejuvenation continues to increase, various dermal fillers, lasers, light source technologies, and RF devices are now being developed4,5,6,7,8, with any number of combination treatments also reported3,9,10,11,12. In the present study, we evaluated the safety and efficacy of a combination regimen of HA filler and a new needle RF device. Owing to several key advantages, HA has become the most widely used filler worldwide. Specifically, HA rarely induces immunogenic reactions and is stable enough to be used in combination treatment regimens, as it has no species or tissue specificity13. Moreover, another advantage of HA is that it is easily degraded by hyaluronidase. The HA filler used in this study, Glytone 3, consists of 23 mg/ml HA in phosphate buffer with mannitol14, which acts as both an antioxidant and a moisturizer15.
RF is different from laser energy and is defined as a nonionizing electromagnetic radiation in a frequency range between 3 kHz to 300 GHz16. RF is also chromophore independent and induces a heat response depending on the electrical properties of the tissue. Initial collagen denaturation within these thermally modified tissues is thought to explain the immediate tissue contraction, with subsequent neocollagenesis further tightening the dermal tissue6,17. The INNOfill device used in this study is a minimally invasive needle RF device newly developed for filler injections; it is capable of delivering high-energy fluencies directly to the dermis.
Several previous studies have evaluated the safety and efficacy of combined treatment regimens with lasers and RF devices9,10,11,12. Specifically, in a study of the effects of RF treatment on soft-tissue fillers in an animal model, England et al.9 suggested that multiple passes of RF treatment directly over filler-injected skin does not promote immediate adverse reactions, nor does it adversely affect the treatment duration of various fillers. Goldman et al.3 also reported that laser, RF, and intense pulsed light treatment can safely be administered immediately after HA treatment in humans.
In the present study, the nasolabial fold was selected as an anatomic site, as each side of the face could be treated by using a different method for easy comparison. The local mass effect of the HA filler lasted between 6 and 12 months, with the duration primarily determined by the degree of movement of the injected site4. As the nasolabial fold frequently moves during facial expressions, the injected filler often spreads and breaks down faster than in other less mobile areas. Therefore, combination therapies are often used to maintain a longer effect. The beveling and fern techniques have since been developed to prevent filler migration to the upper part of the nasolabial fold.
This is the first study to evaluate the safety and efficacy of a new RF needle device that creates autologous containment collagen canals in the dermis and then delivers the filler with the same needle. This new device has several advantages over conventional single modalities. First, the INNOfill device prevents the spread of injected filler material through needle tunneling and the collagen degeneration that occurs around the tunnels. We histologically evaluated the local volume effect and distribution pattern in the tissue, and found that the local volume effect was more evident in subjects treated with filler after treatment with RF than in those treated with filler injections after tunneling (without RF) or filler injections alone. Filler injections after tunneling (without RF) also proved effective, although not as effective as treatment with RF. Whereas the filler generally spreads as particles throughout the dermis, filler particles were observed only in the tunnels in the mid or lower dermis after RF treatment with the INNOfill device. Consequently, the duration of the local mass effect was longer. We thus characterize these tunnels as autologous containment collagen canals, and suggest that such canals represent the core mechanism of the RF needle device. Furthermore, these canals also act as a protective barrier against exogenous oxygen radicals. In this way, the protective and storage capacity of the autologous containment collagen canals likely prolongs the duration of the HA filler effect and reduces the amount of filler required for individual treatments.
RF energy also results in dermal remodeling. As noted above, RF energy clearly reduces the elastic material, reorients the existing elastic material, and induces new collagen formation6. We evaluated the thermal profile of RF and the associated effect on collagen contraction and procollagen induction by using porcine skin and FPCLs. In the study with porcine skin, the heating effect of the needle-delivered RF was confined to the dermis and not associated with any epidermal damage. Procollagen production after treatment with RF was also observed to be time dependent, with a 30-minutes application resulting in a significant difference in the FPCL study. The histologic results from the animal study also demonstrate increased quantities of collagen bundles and elastic fibers. Lastly, the derma scan results from the in vivo study also demonstrate increased brightness at sites treated with the combination regimen, indicating more prominent collagen synthesis.
Finally, patients can easily be treated with both RF and HA filler simultaneously. Most previous combination regimens included serial treatments that are not performed on the same day because of concerns about adverse effect, although Goldman et al.3 reported that combined therapies of laser or RF treatment immediately after filler injection are also safe. Nonetheless, no significant differences have ever been identified between combined therapies and treatment with filler alone. Additionally, previous combination treatment regimens required more time and effort for coordination and treatment delivery than the new needle RF device described in the present study.
There is a rare possibility of adverse and negative effects on the filler treatment because RF energy is delivered before filler treatment and filler materials are not affected by RF energy. HA has minimal immunogenicity and a low rate of adverse events; thus, HA is the most appropriate filler material for combination treatments.
One limitation of treating patients with RF before filler injection is that fine sculpting may become more difficult. Therefore, we recommend that approximately 70% of the filler be injected as part of the combination regimen, whereas the remaining filler be reserved for the treatment of fine wrinkles and recesses by using the linear threading technique.
Although our sample size was small and our histologic study was not performed with human tissue, we evaluated the safety and efficacy of a combination regimen through an in vitro study, an animal study, and an in vivo study. As the local mass effect of the filler was difficult to measure, there is clearly a need for an objective evaluation method of the mass effect, such as use of an imaging device to evaluate the contour lines.
In conclusion, a new combined treatment performed with an RF needle device may represent a safe and effective method for skin rejuvenation, especially in highly mobile areas such as the nasolabial folds. However, further studies with longer follow-up periods and larger sample sizes, as well as head-to-head trials with conventional treatments, are warranted to better assess the efficacy and safety of this new device.
Figures and Tables
 | Fig. 1(A) Novel minimally invasive radiofrequency (RF) device. (B) Procedure protocol. 1. Insertion of the needle along the wrinkle course at variable depths from the superficial to mid dermis, depending on the wrinkle type and the filler used. 2. In situ 360-degree rotation (tunneling) performed to create a virtual canal contextually supplying the RF. 3. The procedure is repeated in retrograde through a multi-prick technique along the wrinkle course. 4. Without removing the needle from the injection site, the needle retraces the course, releasing hyaluronic acid through the linear retrograde technique. This technique yields wrinkle smoothing with a long-lasting effect. |
 | Fig. 2Radiofrequency (RF)-induced collagen contraction and production in fibroblast-populated collagen lattices (FPCLs). (A) FPCL contraction assay diagram. As illustrated, cell-seeded matrices develop isometric tension during an initial attachment period (1~2 days) that later dissipates when lattices are mechanically released. RF treatment performed at six sites on the FPCLs after mechanical release. (B) Plate map of the RF application sites on the FPCLs. At each site, RF was delivered for 1 to 20 minutes per site in a time-dependent manner. (C) Digital images of the contracting FPCLs were captured at 24 h and 48 h after RF application. (D) FPCL contraction diameters after an incubation period of 2 days. All plotted data represent the mean±standard error of the mean (SEM) values for all three FPCLs. Statistical pairwise comparisons were performed by using Student's t-test. Statistical significance (p<0.05) was reached between normal and RF-treated FPCLs. (E) Percent change of FPCL surface area after RF treatment and an incubation period of 2 days. All plotted data represent the mean±SEM values for all three FPCLs. Statistical pairwise comparisons were performed by using Student's t-test. Statistical significance (p<0.05) was reached between normal and RF-treated FPCLs. (F, G) Procollagen production after RF treatment and an incubation period of 2 days. All plotted data represent the mean±SEM values for all three FPCLs. Statistical pairwise comparisons were performed by using Student's t-test. Statistical significance (p<0.05) was reached between normal and RF treated FPCLs. *p<0.05: significant. |
 | Fig. 3(A) Thermal profiles after radiofrequency (RF) application in ex vivo porcine skin. Thermal profiles before (B) and 10 seconds after RF application (C). |
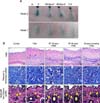 | Fig. 4(A) Volumizing effect from filler injections after radiofrequency (RF) treatment. N: normal, F: filler (Glytone 3) injection, RF30s+F: filler injection immediately after 30 seconds of RF treatment through the tunneling method, RF60s+F: filler injection immediately after 60 seconds of RF treatment through the tunneling method, T30s+F: filler injection immediately after 30 seconds of tunneling without RF treatment. (B) Filler-injected areas are shown without RF or with RF treatment ('*' sign). The filler-injected areas showed small filler particles spread in the dermis, and areas injected with filler after RF or with tunneling showed linear filler distribution in the mid or lower dermis, respectively ('*' sign). The periphery and area around the filler after RF treatment were stained with Masson's trichrome to identify the extracellular matrix components, in particular the dermal collagen. A significantly greater increase in collagen bundles was observed in subjects treated with both filler and RF when compared with those treated with filler alone (blue). Additionally, fibroplasia (red) was also observed in larger quantities after RF treatment when compared with filler injections alone. The tissue specimens were also stained with Verhoeff-Van Gieson to assess the quantity of elastin fibers in the dermis. Classically, long, thin elastin fibers are distributed throughout the papillary and upper reticular dermis. In the results presented here, a change in elastin fiber quality was observed after treatment with RF, with the elastin fibers in the papillary and reticular dermis noted to be markedly shorter ('arrow' sign). |
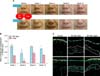 | Fig. 5(A) Photographs of the nasolabial wrinkles of the patients at baseline and at 1, 4, 8, and 12 weeks after either filler injection or filler injection with radiofrequency treatment (RF+filler). (B) Relative wrinkle severity rating scale (WSRS) score at baseline and at 1, 4, 8, and 12 weeks after either filler injection or RF+filler treatment. All plotted data represent the mean±standard error of the mean values for the two patients. Statistical pairwise comparisons were performed by using Student's t-test. Statistical significance was reached (p<0.05) between filler and RF+filler. *p<0.05: significant. (C) Evaluation of collagen production at filler injection sites at 12 weeks. |
ACKNOWLEDGMENT
This work was supported by the Infrastructure Program for New-growth Industries (10044186, Development of Smart Beauty Devices Technology and Establishment of Commercialization Support Center) funded by the Ministry of Trade, Industry, and Energy (Korea).
References
1. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002; 11:398–405.

2. el-Domyati M, el-Ammawi TS, Medhat W, Moawad O, Brennan D, Mahoney MG, et al. Radiofrequency facial rejuvenation: evidence-based effect. J Am Acad Dermatol. 2011; 64:524–535.

3. Goldman MP, Alster TS, Weiss R. A randomized trial to determine the influence of laser therapy, monopolar radiofrequency treatment, and intense pulsed light therapy administered immediately after hyaluronic acid gel implantation. Dermatol Surg. 2007; 33:535–542.

4. Buck DW 2nd, Alam M, Kim JY. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009; 62:11–18.

5. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009; 25:86–94.

6. Hantash BM, Ubeid AA, Chang H, Kafi R, Renton B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009; 41:1–9.

7. Day DJ, Littler CM, Swift RW, Gottlieb S. The wrinkle severity rating scale: a validation study. Am J Clin Dermatol. 2004; 5:49–52.
8. Kim KH, Geronemus RG. Nonablative laser and light therapies for skin rejuvenation. Arch Facial Plast Surg. 2004; 6:398–409.

9. England LJ, Tan MH, Shumaker PR, Egbert BM, Pittelko K, Orentreich D, et al. Effects of monopolar radiofrequency treatment over soft-tissue fillers in an animal model. Lasers Surg Med. 2005; 37:356–365.

10. Shumaker PR, England LJ, Dover JS, Ross EV, Harford R, Derienzo D, et al. Effect of monopolar radiofrequency treatment over soft-tissue fillers in an animal model: part 2. Lasers Surg Med. 2006; 38:211–217.

11. Alam M, Levy R, Pajvani U, Ramierez JA, Guitart J, Veen H, et al. Safety of radiofrequency treatment over human skin previously injected with medium-term injectable soft-tissue augmentation materials: a controlled pilot trial. Lasers Surg Med. 2006; 38:205–210.

12. Park KY, Park MK, Li K, Seo SJ, Hong CK. Combined treatment with a nonablative infrared device and hyaluronic acid filler does not have enhanced efficacy in treating nasolabial fold wrinkles. Dermatol Surg. 2011; 37:1770–1775.

13. Kim JE, Sykes JM. Hyaluronic acid fillers: history and overview. Facial Plast Surg. 2011; 27:523–528.

14. Turlier V, Rouquier A, Black D, Josse G, Auvergnat A, Briant A, et al. Assessment of the clinical efficacy of a hyaluronic acid-based deep wrinkle filler using new instrumental methods. J Cosmet Laser Ther. 2010; 12:195–202.

15. Belda JI, Artola A, García-Manzanares MD, Ferrer C, Haroun HE, Hassanein A, et al. Hyaluronic acid combined with mannitol to improve protection against free-radical endothelial damage: experimental model. J Cataract Refract Surg. 2005; 31:1213–1218.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download