Abstract
Background
A number of reports have been published regarding the use of imiquimod for the treatment of melanoma in situ and metastatic melanoma. Essential steps in the process of melanoma invasion and metastasis include degradation of basement membranes and remodeling of the extracellular matrix by proteolytic enzymes, including matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).
Objective
To evaluate the antiinvasive effect of imiquimod in human malignant melanoma cell lines, SK-MEL-2 and SK-MEL-24, in vitro, and to investigate imiquimod-induced changes in the expression of MMPs and TIMPs.
Methods
Invasiveness of melanoma cell lines following imiquimod treatment was evaluated by invasion assays. In order to investigate the mechanism of the anti-invasive effect of imiquimod, mRNA and protein levels of MMP-2, -9, membrane type 1 (MT1)-MMP, TIMP-1, and -2 were assessed by real-time reverse transcription-polymerase chain reaction, gelatin zymography, and western blotting.
Results
Imiquimod treatment decreased in vitro viability of melanoma cells in a concentration-dependent manner. Imiquimod also elicited a concentration-dependent suppression of invasion in both melanoma cell lines. A concentration-dependent decrease in MMP-2 and MT1-MMP protein levels and a concentration-dependent increase in TIMP-1 and -2 protein levels by imiquimod was observed in both melanoma cell lines. However, expression of MMP-9 protein was increased in SK-MEL-2 but decreased in SK-MEL-24 with increasing imiquimod concentrations. Imiquimod elicited alterations in MMPs and TIMPs mRNA levels that parallel the observed changes in protein levels.
Imiquimod is an immune response modulator, which is used for the treatment of actinic keratoses, superficial basal cell carcinomas, and genital and perianal warts. Imiquimod has also been used in the treatment of melanoma in situ and metastatic melanoma in patients with confounding morbidities who are not considered candidates for surgery, with extensive disease or disease in areas that are not amenable to surgery1,2,3,4,5.
Melanoma is a well-known tumor that tends to metastasize, rather than grow locally. During the process of tumor invasion, essential steps include the degradation of basement membranes and remodeling of the extracellular matrix (ECM) by proteolytic enzymes such as matrix metalloproteinases (MMPs) under regulation by tissue inhibitors of metalloproteinases (TIMPs). MMPs, particularly MMP-2 and MMP-9, are key enzymes known to degrade the components of surrounding ECM during cancer invasion and metastasis. Melanoma cells express a number of MMPs and TIMPs6.
So far, there has only been one case report investigating the changes in the expression of factors involved in melanoma metastasis after treatment with imiquimod7. In that study, a skin metastatic lesion was biopsied before and after treatment with imiquimod, and the expression of the molecular regulators investigated using real-time reverse transcription-polymerase chain reaction (RT-PCR). Following imiquimod treatment, the expression of TIMP-1, KiSS-1, and MMP-1 was up-regulated, that of MMP-2 was not altered, and MMP-9 expression was dramatically decreased. These findings suggest that imiquimod could repress metastasis and inhibit melanoma invasion7.
The aim of the current study was to evaluate the anti-invasive effects of imiquimod in vitro against human malignant melanoma cell lines. Additionally, this study also investigated imiquimod-induced changes in the expression of key ECM-degrading enzymes MMP-2, -9, and membrane type 1 MMP (MT1-MMP), along with their inhibitors TIMP-1 and -2. The targets of this investigation are key enzymes known to degrade the surrounding ECM components during cancer invasion and metastasis.
Melanoma cell lines, SK-MEL-2 and SK-MEL-24, as well as the HT1080 cell line (used as a positive control), were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained using routine procedures. SK-MEL-2 and SK-MEL-24 were maintained in Eagle's minimal essential medium (Lonza, Basel, Switzerland) containing 10% fetal bovine serum (FBS; Lonza) and supplemented with 100 units/ml penicillin and 100 mg/ml streptomycin. HT1080 cells were maintained in RPMI-1640 (Lonza) containing 10% FBS and supplemented with 100 units/ml penicillin and 100 mg/ml streptomycin.
SK-MEL-2 and SK-MEL-24 cells were harvested in the exponential growth phase and seeded in a 96-well flatbottom tissue culture plate at a concentration of 1×104 cells/100 µl in each well. Cells were allowed to grow and stabilize for 24 hours. Subsequently, the cells were treated with a range of concentrations (5~200 µg/ml) of imiquimod (InvivoGen, San Diego, CA, USA) prepared in a complete medium or cultured for a range of incubation times (6 hours~3 days). Each treatment was performed in three replicates wells. After incubation, 10 µl of WST-1 reagent EZ-CyTox (Daeil Lab, Seoul, Korea) was added to each well, followed by incubation for 4 hours at 37℃. Optical density was measured using enzyme-linked immunosorbent assay plate reader (Molecular Devices; Spectra Max 190 with Soft max Pro, Sunnyvale, CA, USA) at 450 nm with a reference wavelength of 690 nm. Cell viability was plotted as a percentage of untreated control. Results are expressed as mean±standard error of the mean and are representative of three independent experiments. The half maximal inhibitory concentration (IC50) was determined from the dose-effect curve as the drug concentration that decreased cell viability to 50% of the original value.
A modified version of the standard transwell filter assay commonly used for evaluating invasion was performed. Transwell filters (diameter, 6.5 mm; pore size, 8 µm; Falcon, Becton Dickinson and Company, Franklin Lakes, NJ, USA) were coated on the lower side with 8 µg/µl matrigel and placed on a 24-well plate containing medium supplemented with 0.1% bovine serum albumin (BSA; USB, Cleveland, OH, USA). SK-MEL-2 and SKMEL-24 were harvested with a cell dissociation solution (Sigma-Aldrich, St Louis, MO, USA) and suspended in medium with 1% BSA. Cancer cells (1×105 cells) were added to the upper compartment of a transwell chamber and treated with 0, 10, and 30 µg/ml imiquimod added to the upper compartment for 24 hours at 37℃. After 24 hours, non-migrated cells on the upper side of the membrane were removed with a cotton swab, and migrated cells on the bottom surface of the membrane were fixed in 3.7% paraformaldehyde in phosphate buffered saline (Lonza) and stained with crystal violet for 10 minutes at room temperature. Cell migration was quantified by counting the number of cells in three inserts. Data were expressed as the average number of cells per insert.
SK-MEL-2, SK-MEL-24, and HT1080 cell lines were cultured in serum-free media for 24 hours, and the conditioned media was concentrated using VIVASPIN 20 (Sartorius Stedim Biotech GmbH, Goettingen, Germany). An equal volume of sample buffer was added to the concentrated media before loading on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel impregnated with 0.1% gelatin. After electrophoresis, the gel was rinsed with renaturing buffer for 1 hour and incubated in a developing buffer overnight at 37℃. After incubation, the gel was stained with 0.05% Coomassie brilliant blue R-250 (Amresco, Cleveland, OH, USA) and de-stained. MMP-2 and MMP-9 were detected as transparent bands. The HT1080 cell line was used as a positive control.
To evaluate the expression of MT1-MMP, whole-cell protein extracts were prepared from SK-MEL-2, SK-MEL-24, and HT1080 cells treated with a range of concentrations of imiquimod (0, 5, 10, and 30 µg/ml) for 24 hours. Protein content was determined by the bicinchoninic acid (BCA) assay method. Aliquots of each sample (40 µg of total protein per sample) were fractionated by 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). After blocking with 5% skimmed milk, the membranes were incubated with polyclonal antibody against MT1-MMP (1 : 500 dilution; Abcam Inc., Cambridge, UK) and monoclonal antibody against actin (1 : 4,000 dilution; Santa Cruz biotechnology, Santa Cruz, CA, USA) at 4℃ overnight. After washing with tris-buffered saline with Tween 20 (TBST), the membranes were incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (1 : 2,000 dilution; Cell Signaling, Beverly, MA, USA) and goat anti-mouse IgG antibody (1 : 2,000 dilution; Cell Signaling).
To evaluate the expression of TIMP-1 and -2, cell culture supernatants were collected from melanoma cell lines and HT1080 cell line. Samples of conditioned medium (7 ml) were precipitated with 10% trichloroacetic acid (TCA) at -20℃ overnight. Following centrifugation at 4,300 rpm for 20 minutes at 4℃, the pellet was washed twice with ice-cold acetone, dried at 37℃, and dissolved in 100 µl of 2× SDS sample buffer. An aliquot of each sample (20 µl per sample) was fractionated by 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (GE Healthcare). After blocking with 5% skimmed milk, membranes were incubated with monoclonal antibodies against TIMP-1 (1 : 500 dilution; Abcam Inc.) and TIMP-2 (1 : 500 dilution; Abcam Inc.) at 4℃ overnight. After washing with TBST, membranes were incubated with peroxidase-conjugated goat anti-mouse IgG antibody (1 : 2,000 dilution; Cell Signaling). Chemiluminescence reagents (ECL Plus Western Blotting Detection Reagents; GE Healthcare) were used for the visualization of the labeled protein bands. The HT1080 cell line was used as a positive control.
SK-MEL-2, SK-MEL-24, and HT1080 cell lines were treated with a range of concentrations of imiquimod (0, 5, 10, and 30 µg/ml) for 24 hours. Total cellular RNA was purified from cancer cells using Trizol® reagent (Gibco BRL; Life Technologies, Carlsbad, CA, USA). After evaluation by gel electrophoresis, the RNA of each sample was stored at -80℃ until use. The total RNA was converted to single- stranded cDNA using a High Capacity kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. The resulting cDNA was stored at -20℃. Measurements of mRNA levels were based on SYBR® Green PCR Master Mix (Applied Biosystems) using the 7500 Real-Time PCR System (Applied Biosystems). Primers were designed using Primer Express® Software v3.0 (Applied Biosystems). Sense and antisense primers for MMP-2 were 5'-GCAGACATCGTCATCCAGTTTG-3' and 5'-CCGTCCTTCCCGTCGAA-3', respectively. Sense and antisense primers for MMP-9 were 5'-GATGACGATGAGCTATGGACCTT-3' and 5'-TCGGCGTTCCCATACTTCAC-3', respectively. Other primers and probes were synthesized by Assay-on-Demand products (Applied Biosystems): MT1-MMP (HS 00237119), TIMP-1 (HS 0017-1557), TIMP-2 (HS 00234278), and GAPDH (HS 999-99905). RT-PCR was performed in triplicate 20 µl reaction volumes consisting of 10 µl 2× TaqMan® Gene Expression Master Mix (Applied Biosystems), 1 µl 20× Gene Expression Assay Mix, and 9 µl cDNA sample diluted in RNase-free water. Two-step PCR cycling was carried out as follows: 50℃ for 2 minutes and 95℃ for 10 minutes (1 cycle), 95℃ for 15 seconds, and 60℃ for 1 minute (50 cycles). Relative expression of mRNA was calculated using a relative quantification method, which determines the relative expression of a target gene in comparison with a reference gene.
A cell viability assay was performed. Melanoma SK-MEL-2 and SK-MEL-24 cells were treated with imiquimod and a concentration- and incubation time-dependent inhibition of cell viability was observed. Fig. 1A shows the concentration-dependent decrease in viability of SK-MEL-2 cells over 24 hours of incubation. The IC50 value calculated from the dose-effect curve was found to be 56.32 µg/ml. The viability of SK-MEL-24 cells also decreased with increasing imiquimod concentrations over 24 hours of incubation (Fig. 1B). The IC50 value of SK-MEL-24 cells calculated from the dose-effect curve was 62.36 µg/ml.
To evaluate the anti-invasive effect of imiquimod over a range of concentrations in the two melanoma cell lines, a modified version of the standard transwell filter assay for invasion was performed. As shown in Fig. 2A~C and 2E~G, the migratory activity and invasion potential of the two melanoma cell lines were significantly suppressed by increasing concentrations of imiquimod over 24 hours of incubation. Fig. 2D and H show the percentages of relative cell counts after the invasion assay. The number of invading melanoma cells was significantly decreased by the increasing concentrations of imiquimod. Invading cells were 31.57% and 55.96% lower in SK-MEL-2 cells treated with 10 and 30 µg/ml imiquimod, respectively, as compared to the non-treated SK-MEL-2 cells (Fig. 2D). Invasion of SK-MEL-24 cells was significantly decreased by 43.87% and 67.02% after treatment with 10 and 30 µg/ml imiquimod, respectively, as compared with non-treated SK-MEL-24 cells (Fig. 2H).
Gelatin zymography and real-time quantitative RT-PCR were performed to determine whether the function of imiquimod in control of melanoma invasiveness is dependent on MMP expression and activity.
In gelatin zymography, only the inactive form of MMP-2 was detected in the SK-MEL-2 cell line (Fig. 3A), while both the inactive and active forms of MMP-2 were observed in the SK-MEL-24 cells (Fig. 3B). Expression of MMP-2 decreased following incubation with increasing concentrations of imiquimod in both cell lines (Fig. 3A, B). However, the expression of MMP-9 was different in the SK-MEL-2 and SK-MLE-24 cell lines. Inactive and active forms of MMP-9 were observed in SK-MEL-2 cell line (Fig. 3C), while only its inactive form was observed in SK-MEL-24 cells (Fig. 3D). SK-MEL-2 cells showed a concentration-dependent increase in MMP-9 expression following imiquimod incubation (Fig. 3C). However, expression of MMP-9 decreased with increasing imiquimod concentrations in SK-MEL-24 cell lines (Fig. 3D).
In real-time quantitative RT-PCR investigations, expression of MMP-2 and -9 mRNA showed a pattern similar to the one observed with MMP-2 and -9 proteins in both cell lines. In SK-MEL-2 cells, MMP-2 mRNA showed a significant concentration-dependent decrease following treatment with imiquimod at all concentrations (p<0.05; Fig. 3E). In SK-MEL-24 cells, mRNA expression of MMP-2 also exhibited concentration-dependent decreases following imiquimod treatment. Significantly decreased expression of MMP-2 mRNA was detected in SK-MEL-24 cells following incubations with 10 and 30 µg/ml imiquimod, compared with untreated cells (p<0.05; Fig. 3F). MMP-9 mRNA expression in SK-MEL-2 cells tended to increase with increasing concentrations of imiquimod, but the relationship was not statistical significant (Fig. 3G). In SK-MEL-24 cells, significantly decreased mRNA expression of MMP-9 was observed following 30 µg/ml imiquimod (p<0.05; Fig. 3H).
To investigate the changes in MT1-MMP protein expression, western blot analysis was performed on the whole-cell protein extracts of melanoma cells treated with imiquimod (0~30 µg/ml). Both inactive and active MT1-MMP proteins were detected in both melanoma cell lines. Expression of MT1-MMP proteins was found to be inversely proportional to the concentration of imiquimod in both cell lines (Fig. 4A, B).
A significant concentration-dependent decrease in MT1-MMP mRNA was observed in SK-MEL-2 cells following treatment with imiquimod at all concentrations (p<0.05; Fig. 4C). In SK-MEL-24 cells, mRNA expression of MT1-MMP was decreased in a concentration-dependent manner following imiquimod treatment, with a statistically significant decrease observed at 30 µg/ml (p<0.05; Fig. 4D).
Whole protein extracts obtained from conditioned media of two melanoma cell lines treated with 0 to 30 µg/ml of imiquimod were collected and concentrated. TIMP-1 and -2 proteins were detected in both melanoma cell lines following treatment with all concentrations of imiquimod. Expression of TIMP-1 protein showed a tendency to increase with increasing imiquimod concentration in both cell lines (Fig. 5A, B). Elevated TIMP-2 protein expression was also observed in concentration-dependent patterns in both cell lines (Fig. 5C, D).
In real-time quantitative RT-PCR investigations, changes in TIMP-1 and -2 mRNA levels showed a pattern similar to that observed with TIMP-1 and -2 protein levels in both cell lines. Additionally, mRNA expression of TIMP-1 in SK-MEL-2 cells tended to increase with increasing concentrations of imiquimod, but the imiquimod-induced changes did not reach statistical significance (Fig. 5E). In SK-MEL-24 cells, mRNA expression of TIMP-1 was elevated with increasing imiquimod in a concentration-dependent manner, with statistically significant differences observed at 30 µg/ml of imiquimod (p<0.05, compared to control cells; Fig. 5F). TIMP-2 mRNA expression tended to increase with the concentration of imiquimod in SK-MEL-2 cells, without reaching statistical significance (Fig. 5G). Lastly, mRNA expression of TIMP-2 in SK-MEL-24 cells was significantly increased in a concentration-dependent manner following treatment with all concentrations of imiquimod (p<0.05; Fig. 5H).
Previous studies investigating imiquimod focused mainly on its antitumor effects, elicited through the induction of profound cellular tumor-directed immune responses by inflammatory cells8,9 or through direct pro-apoptotic activity against tumor cells8,10,11. This study demonstrated that imiquimod suppresses the invasion of melanoma cells in a concentration-dependent manner.
Cancer invasion and metastasis occurs through degradation of stromal connective tissue and the components of the basement membrane12,13. In order to invade and metastasize, tumor cells adhere to the ECM and degrade it through the secretion and activation of multiple proteolytic enzymes, particularly MMPs14. Increased MMP activity is related to tumor growth, invasion, and angiogenesis15,16,17. TIMPs are specific inhibitors that regulate the local activities of MMPs18,19,20 and include TIMP-1, -2, -3, and -415,21,22. Melanoma cells show expression of MMP-1, -2, -9, -13, and MT1-MMP, as well as inhibitors TIMP-1, -2, and -36,23.
Among MMPs, MMP-2 and its inhibitor TIMP-2 have been found to control degradation of type IV collagen, which is the main component of the basement membrane. MMP-2 is, therefore, an important enzyme known to degrade the surrounding ECM components during cancer invasion and metastasis24,25. A relationship between MMP-2 expression and prognosis of melanoma has been reported. An increase in MMP-2 expression was found to correlate with hematogenous metastasis of melanoma23,26,27. In the current study, imiquimod decreased the expression of MMP-2 in a concentration-dependent manner in both melanoma cell lines studied. This observed effect may be associated with the imiquimod-mediated suppression of melanoma invasion and metastasis.
MMP-9 enzyme has the ability to degrade type IV collagen, plays a key role in the early stages of tumor invasion and metastasis, and is inhibited by TIMP-128. Imbalance in the production of MMP-9 and TIMP-1 is involved in the regulation of ECM degradation, thereby affecting tumor invasion and metastasis29. The exact role of MMP-9 in melanoma progression is controversial. Expression of MMP-9 in primary melanoma was observed in the horizontal growth phase, but not in the vertical growth phase23,30. On the other hand, expression of MMP-9 was observed in a melanoma cell line derived from advanced primary melanoma, but not in a melanoma cell line derived from early primary melanoma31. Tumor cells from spontaneous lymph node and pulmonary metastases were found to express mostly MMP-9, whereas no MMP-9 expression was detected in cells from experimental metastases32. In our current study, expression of MMP-9 in the two melanoma cell lines studied was altered in the opposite directions by the incubation with imiquimod. Expression of MMP-9 was increased in the SK-MEL-2 cell line, but decreased in the SK-MEL-24 cell line with increasing imiquimod concentrations. The observed difference in the effect may be related to the origin of the melanoma cell lines. The SK-MEL-2 cell line was derived from a skin metastasis of melanoma, while the SK-MEL-24 cell line was derived from a lymph node metastasis of melanoma. Selected tumor cells in different environments may exhibit different properties, which influence their invasiveness and capacity to metastasize to different organs; therefore, metastases to various organs may occur by different mechanisms. These unique characteristics of each cell could be reflected in their response to imiquimod. Further studies are warranted to determine the biochemical factors involved in the control of MMP-9 expression in melanoma cell lines. Expression of TIMP-1 showed a concentration-dependent increase following imiquimod treatment in both cell lines. In this study, suppression of the invasiveness of the two melanoma cell lines was independent of changes in MMP-9 expression. Our findings, therefore, suggest that imiquimod-induced increases in TIMP-1 may play a more important role in the control of melanoma cell invasion than the changes in MMP-9.
MT1-MMP is a membrane-bound MMP that plays a dual role in degradation of ECM in the tumor invasion process33,34. MT1-MMP can activate proMMP-2 and degrade a number of ECM components35,36,37, thereby promoting tumor invasion and metastasis. In primary and metastatic melanoma, expression of MMP-2 and MT1-MMP enzymes was found to be increased. Melanoma cells co-expressing MMP-2 and MT1-MMP were often located at the front of the melanoma invasion process. Melanoma cells expressing MMP-2 also showed co-expression of MT1-MMP and TIMP-223,38. TIMP-2 serves to bridge the interaction between MMP-2 and MT1-MMP. At low TIMP-2 concentrations, proMMP-2 is activated by free MT1-MMP. At high intracellular TIMP-2 concentrations, most MT1-MMP forms complexes with TIMP-2, which function as receptors for proMMP-2. The ternary complex of MT1-MMP/TIMP-2/proMMP-2 interacts with the adjacent free-MT1-MMP altering proMMP-2 activation. Overall, this process partially decreases the activation of proMMP-239,40. In our current study, imiquimod treatment resulted in a concentration-dependent decrease in MMP-2 and MT1-MMP levels, while TIMP-2 levels were observed to increase with increasing concentrations of imiquimod. These changes may act synergistically to suppress the invasiveness of melanoma cells mediated through MMPs and TIMPs.
Over-expression of TIMP-1, -2, and -3 was observed during the progression of melanoma41. Over-expression of TIMP-1 and -2 reduces the experimental metastasis of melanoma42,43. TIMPs were also reported to exhibit apoptosis-inducing properties44. In human melanoma cell lines, expression of TIMP-1 and -2 was increased by imiquimod treatment in a dose-dependent manner. Increases in TIMP-1 and -2 levels can directly suppress the activity of MMPs in melanoma, thereby suppressing melanoma invasion and metastasis.
In the future, anti-cancer treatments focused on regulating MMP and TIMP activity warrant further development for clinical use. The results of our current study suggest that imiquimod may be used to attenuate cancer progression by suppressing cancer invasion and metastasis. However, further investigations evaluating the anti-invasive and anti-metastatic effects of imiquimod, including in vivo studies in human patients and murine disease models, are needed.
In conclusion, imiquimod induced a concentration-dependent suppression of invasion in human malignant melanoma cell lines. Incubation with imiquimod also caused concentration-dependent alterations in the expression of MMPs and TIMPs. These results suggest that imiquimod may elicit an anti-invasive effect on human melanoma cells by regulating the balance of MMPs and TIMPs.
Figures and Tables
 | Fig. 1
In vitro cell viability of melanoma cell lines SK-MEL-2 (A) and SK-MEL-24 cells (B) following incubation with a range of concentrations of imiquimod. The graphical representation of the dose-effect relationship depicts a concentration-dependent inhibition in the percent viability of cells following imiquimod treatment (IC50 values: 56.32 µg/ml for SK-MEL-2, 62.36 µg/ml for SK-MEL-24). |
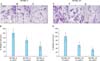 | Fig. 2Invasion assay performed in SK-MEL-2 and SK-MEL-24 cell lines following imiquimod treatment. Suppression of SK-MEL-2 cell migration and invasion following incubation with a range of concentrations of imiquimod (A) 0 µg/ml, (B) 10 µg/ml, and (C) 30 µg/ml imiquimod (A~C: Coomassie stain, ×200). (D) The percentage of invasive SK-MLE-2 melanoma cells was significantly decreased with increasing concentrations of imiquimod (*p<0.05). Suppression of SK-MEL-24 cell migration and invasion following treatment with a range of concentrations of imiquimod (E) 0 µg/ml, (F) 10 µg/ml, and (G) 30 µg/ml imiquimod (E~G: Coomassie stain, ×200). (H) The percentage of invasive SK-MLE-24 melanoma cells was significantly decreased with increasing concentrations of imiquimod (*p<0.05). Con A: concanavalin A. |
 | Fig. 3Expression of matrix metalloproteinase (MMP)-2 and -9 protein and mRNA in SK-MEL-2 and SK-MEL-24 cell lines following incubation with imiquimod. Expression of MMP-2 decreased with increasing concentrations of imiquimod in SK-MEL-2 cells (A) and SK-MEL-24 cells (B). (C) MMP-9 expression in SK-MEL-2 cells was increased by imiquimod treatment in a dose-dependent manner. (D) However, expression of MMP-9 was dose-dependently decreased in SK-MEL-24 cells treated with imiquimod. Conditioned media of the HT1080 cell line was used as a positive control. Relative mRNA expression of MMP-2 in SK-MEL-2 (E) and SK-MLE-24 (F) cells and MMP-9 in SK-MEL-2 (G) and SK-MLE-24 (H) cells, following incubation with a range of concentrations of imiquimod (5, 10, and 30 µg/ml), compared to controls (0 µg/ml). *p<0.05 vs. control. |
 | Fig. 4Expression of membrane type 1 matrix metalloproteinase (MT1-MMP) protein and mRNA in SK-MEL-2 and SK-MEL-24 cell lines following treatment with imiquimod. Expression of MT1-MMP proteins in SK-MEL-2 (A) and SK-MEL-24 (B) cell lines was dose-dependently decreased with increasing concentrations of imiquimod. Relative mRNA expression of MT1-MMP in SK-MEL-2 (C) and SK-MEL-24 (D) cell lines, following treatment with a range of imiquimod concentrations (5, 10, 30 µg/ml), compared to controls (0 µg/ml). Con A: concanavalin A. *p<0.05 vs. control. |
 | Fig. 5Expression of tissue inhibitors of metalloproteinase (TIMP)-1 and -2 protein and mRNA in SK-MEL-2 and SK-MEL-24 cell lines following incubation with imiquimod. Expression of TIMP-1 protein was dose-dependently increased by imiquimod in SK-MEL-2 (A) and SK-MEL-24 cells (B). Expression of TIMP-2 protein was also dose-dependently elevated by imiquimod in SK-MEL-2 (C) and SK-MEL-24 cells (D). Relative mRNA expression of TIMP-1 in SK-MEL-2 (E) and SK-MLE-24 (F) cells and TIMP-2 in SK-MEL-2 (G) and SK-MLE-24 (H) cells, following treatment with a range of concentrations of imiquimod (5, 10, and 30 µg/ml), compared to controls (0 µg/ml).
*p<0.05 vs. control. |
ACKNOWLEDGMENT
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2009 (6-2009-0089) and research grant from Stiefel in 2009.
References
1. van Meurs T, van Doorn R, Kirtschig G. Recurrence of lentigo maligna after initial complete response to treatment with 5% imiquimod cream. Dermatol Surg. 2007; 33:623–626.

2. Wolf IH, Cerroni L, Kodama K, Kerl H. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol. 2005; 141:510–514.

3. Naylor MF, Crowson N, Kuwahara R, Teague K, Garcia C, Mackinnis C, et al. Treatment of lentigo maligna with topical imiquimod. Br J Dermatol. 2003; 149:Suppl 66. 66–70.

4. Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008; 34:147–151.

5. Woodmansee CS, McCall MW. Recurrence of lentigo maligna and development of invasive melanoma after treatment of lentigo maligna with imiquimod. Dermatol Surg. 2009; 35:1286–1289.

6. Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000; 115:337–344.

7. Hesling C, DIncan M, Mansard S, Franck F, Corbin-Duval A, Chèvenet C, et al. In vivo and in situ modulation of the expression of genes involved in metastasis and angiogenesis in a patient treated with topical imiquimod for melanoma skin metastases. Br J Dermatol. 2004; 150:761–767.

8. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002; 3:196–200.

9. Schön M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J, et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003; 95:1138–1149.

10. Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: an overview. Br J Dermatol. 2003; 149:Suppl 66. 53–56.

11. Schön MP, Wienrich BG, Drewniok C, Bong AB, Eberle J, Geilen CC, et al. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J Invest Dermatol. 2004; 122:1266–1276.

12. Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003; 20:203–213.
13. Liotta LA, Rao CN, Wewer UM. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986; 55:1037–1057.

14. Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002; 3:207–214.

15. Sun J. Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells. J Signal Transduct. 2010; 2010:985132.

16. Gokaslan ZL, Chintala SK, York JE, Boyapati V, Jasti S, Sawaya R, et al. Expression and role of matrix metalloproteinases MMP-2 and MMP-9 in human spinal column tumors. Clin Exp Metastasis. 1998; 16:721–728.
17. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010; 141:52–67.

18. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92:827–839.
19. Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997; 74:111–122.
20. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283.

21. Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005; 11:97–103.

22. Stetler-Stevenson WG, Liotta LA, Kleiner DE Jr. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993; 7:1434–1441.

23. Hofmann UB, Houben R, Bröcker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005; 87:307–314.

24. Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980; 284:67–68.

25. Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995; 270:5331–5338.
26. Väisänen A, Tuominen H, Kallioinen M, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (72 kD type IV collagenase) expression occurs in the early stage of human melanocytic tumour progression and may have prognostic value. J Pathol. 1996; 180:283–289.

27. Väisänen A, Kallioinen M, Taskinen PJ, Turpeenniemi-Hujanen T. Prognostic value of MMP-2 immunoreactive protein (72 kD type IV collagenase) in primary skin melanoma. J Pathol. 1998; 186:51–58.

28. Pyke C, Ralfkiaer E, Tryggvason K, Danø K. Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol. 1993; 142:359–365.
29. Sakata K, Shigemasa K, Nagai N, Ohama K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol. 2000; 17:673–681.

30. van den Oord JJ, Paemen L, Opdenakker G, de Wolf-Peeters C. Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am J Pathol. 1997; 151:665–670.
31. MacDougall JR, Bani MR, Lin Y, Rak J, Kerbel RS. The 92-kDa gelatinase B is expressed by advanced stage melanoma cells: suppression by somatic cell hybridization with early stage melanoma cells. Cancer Res. 1995; 55:4174–4181.
32. Hofmann UB, Eggert AA, Blass K, Bröcker EB, Becker JC. Expression of matrix metalloproteinases in the microenvironment of spontaneous and experimental melanoma metastases reflects the requirements for tumor formation. Cancer Res. 2003; 63:8221–8225.
33. d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997; 250:751–757.
34. Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002; 62:580–588.
35. Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000; 149:1309–1323.

36. Iida J, Wilhelmson KL, Price MA, Wilson CM, Pei D, Furcht LT, et al. Membrane type-1 matrix metalloproteinase promotes human melanoma invasion and growth. J Invest Dermatol. 2004; 122:167–176.

37. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998; 273:871–880.

38. Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000; 191:245–256.

39. Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008; 27:57–66.

40. Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, et al. Homophilic complex formation of MT1-MMP facilitates pro-MMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001; 20:4782–4793.

41. Henriet P, Blavier L, Declerck YA. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS. 1999; 107:111–119.

42. Montgomery AM, Mueller BM, Reisfeld RA, Taylor SM, DeClerck YA. Effect of tissue inhibitor of the matrix metalloproteinases-2 expression on the growth and spontaneous metastasis of a human melanoma cell line. Cancer Res. 1994; 54:5467–5473.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download