Abstract
Background
The effects of the Notch signaling pathway in fibroproliferative skin diseases have not been fully elucidated.
Objective
The aim of this study was to investigate the expression of activated Notch signaling molecules in various skin fibroproliferative diseases.
Methods
Immunohistochemical analysis of Notch intracellular domain (NICD) expression in keloid, hypertrophic scar, morphea, dermatofibroma, and normal control skin specimens was performed, and the clinical characteristics of patients with various skin fibroproliferative diseases were analyzed.
Results
NICD was highly expressed in fibroblasts of keloids and moderately to highly expressed in hypertrophic scars and dermatofibromas, whereas low or no expression was detected in the fibroblasts of normal skin specimens and morpheas. NICD was constitutively expressed in keratinocytes, endothelial cells, and immune cells in normal skin specimens.
Notch signaling influences cell fate determination by modulating cell proliferation, differentiation, apoptosis, migration, and invasion1. The Notch family is composed of 4 Notch receptors (Notch-1-Notch-4), and 5 Notch ligands, including Jagged-1 (JAG-1), JAG-2, Delta-like-1, Delta-like-3, and Delta-like-42. Notch activation results in the following mechanisms: proteolytic cleavage of the Notch receptor by a metalloprotease ADAM17; formation of the γ-secretase complex; and the subsequent release of the Notch intracellular domain (NICD), which is then translocated to the nucleus to activate specific target genes such as hes-13,4,5.
The pathologic activation of Notch signaling has been implicated in the pathogenesis of various malignancies and fibrotic diseases, such as systemic sclerosis and idiopathic pulmonary fibrosis6,7,8. The inhibition of Notch signaling has been adopted in clinical trials to treat several malignant tumors such as gastrointestinal neuroendocrine tumors, metastatic thyroid cancer, and advanced breast cancer2. Recent studies have demonstrated the anti-fibrotic effects of Notch signaling inhibition in vitro and genetic inhibition of Notch signaling in murine fibrosis models9,10. Transforming growth factor β1 (TGF-β1) is a principal fibrogenic cytokine that promotes the production of the extracellular matrix and tissue fibrosis11. The activation of the TGF-β signaling pathway is considered to be the main pathogenesis of fibrotic skin diseases such as keloids11. There is evidence that the Notch signaling pathway exerts its effects via upstream or downstream signaling transduction of the TGF-β signaling pathway6,12. A recent study showed that NICD and hes-1 levels increased in skin fibroblasts stimulated by TGF-β in patients with systemic sclerosis9,10. Inhibiting the activation of the Notch receptor attenuates TGF-β-induced α-smooth muscle actin expression6
The role of the Notch signaling pathway in fibroproliferative skin diseases has not been fully elucidated. Therefore, the aim of this study was to investigate NICD expression in normal skin specimens and fibroproliferative skin diseases, including keloids, hypertrophic scars, morpheas, and dermatofibromas.
This study was approved by the ethical committee of The Catholic University of Korea, and all patients signed an informed consent. The study was conducted according to the Declaration of Helsinki principles.
The following formalin-fixed, paraffin-embedded specimens were retrieved: 5 normal human tissues, 5 morpheas, 5 hypertrophic scars, 5 dermatofibromas, and 5 keloids. The clinical information of patients is described in Table 1.
The specimens after fixation in 4% paraformaldehyde were embedded in paraffin for histological or immunohistochemical examinations. All immunohistochemical stainings were performed using a Dako Envision kit (Dako Cytomation Co., Glostrup, Denmark) according to the manufacturer's instructions, using a commercially available prediluted monoclonal antibody against the Notch-1 (Abcam) antigen at a 1 : 200 dilution. Immunohistochemical analysis was performed on samples (Table 1, 2), and the degree of expression was semiquantitatively graded as follows: +, 1% to 19% positive; ++, 20% to 79% positive; +++, 80% to 100% positive. Two independent dermatopathologists scored the samples of 3 high-powered fields per section, and the average score was calculated. All statistical analyses were performed using SPSS software ver. 15.0.1 (SPSS Inc., Chicago, IL, USA).
NICD in keloid and hypertrophic scar fibroblasts showed moderate to high immunoreactivity; similar observations were noted in keratinocytes, endothelial cells, and immune cells (Fig. 1, Fig. 2, Fig. 3, Table 1). In contrast, the extracellular matrix did not express NICD in both tumors. The pattern of NICD expression showed diffuse immunoreactivity in almost all fibroblastic foci of keloids and hypertrophic scars (Fig. 1A, B). Two patients presented recurrent keloids and hypertrophic scars after laser treatment and excision, respectively. Specimens from both these patients exhibited moderate expression of fibroblast NICDs. NICD expression patterns did not vary according to disease duration, site of lesion, and recurrence history.
Dermatofibromas also showed a similar degree of immunoreactivity for NICD in the tumor cells as well as in keloids and hypertrophic scars (Fig. 1C, 2D, 2E, 3, Table 1). Moderate to high immunoreactivity for NICD was detected in the overlying epidermal proliferation but was not detected in the grenz zone (Fig. 1C). The NICD expression pattern showed diffuse immunoreactivity in fibrohistiocytic proliferation arranged in a storiform pattern in dermatofibromas (Fig. 1C, 2D). The proliferation of fibroblast-like spindle cells, histiocytes, and vessels were uniformly stained for NICD (Fig. 2D, E).
NICD expression was generally low in morphea fibroblasts, whereas high expression was detected in keratinocytes and endothelial cells (Fig. 1D, 2F, 3, Table 1). Interestingly, inflammatory infiltrate in a specimen of early morphea lesion showed moderate immunoreactivity for NICD (Fig. 2G). The intensity of the immunopositivity of NICD varied at varied with areas in the same specimens in morphea tissue.
NICD expression was generally low or not detected in fibroblasts of normal control cells, but high expression was detected in keratinocytes of the epidermis, hair follicles, sebaceous and eccrine glands, and endothelial cells (Fig. 1E, 2H, 3, Table 1). NICD was highly stained in the upper epidermal layer than in the basal layer (Fig. 1E). Physiological perivascular infiltrating immune cells showed high expression of NICD (Fig. 2H).
In the present study, NICD expression levels were determined by immunohistochemical analysis of normal skin, keloid, hypertrophic scar, morphea, and dermatofibroma specimens. Keloids and hypertrophic scars are considered to be fibroblastic or myofibroblastic tumors13. Keloid and hypertrophic scar specimens showed moderate to high expression of NICD. Dermatofibroma, a representative fibrohistiocytic tumor, also exhibited moderate to high NICD expression in all tumor components. NICD expression was generally low or not detected in normal fibroblasts, and fibroblasts from patients with morphea were strongly stained compared to the normal cells; however, the proportion of NICD-immunopositive cells in normal fibroblasts was much lower than that of keloids, hypertrophic scars, and dermatofibromas. In contrast, significant staining of NICD in fibroblasts was observed only in various fibroproliferative skin diseases, and the accumulation of NICD in keratinocytes and endothelial cells was also observed in fibroproliferative diseases as well as normal controls.
The Notch signaling pathway is involved in the normal wound healing process by modulating keratinocytes, fibroblasts, and endothelial cells14. Keloids and hypertrophic scars have been considered to be aberrant wound healing consquences11. The significant upregulation of the NICD in keloid fibroblasts observed in the present study is consistent with previous studies9,10,14. Keloid fibroblasts express NICD-1 (54.5%), NICD-2 (38.6%), and JAG-1 at mRNA and protein levels14. The inhibition of Notch signaling via JAG-1 knockdown led to the inhibition of proliferation, migration, and invasion properties of keloid fibroblasts14. Dees et al.9 reported that significant staining of NICD in keloid and hypertrophic scar fibroblasts was also observed in specimens from patients with systemic sclerosis. However, our results showed that the immunoreactivity for NICD in keloids and hypertrophic scars was much higher than that in morphea. These results may be attributed to the fact that the proliferative, migratory, and invasive potential of myofibroblasts and fibroblasts in keloids, hypertrophic scars, and dermatofibromas are higher than those in morphea. Although morphea is considered to be a localized form of scleroderma, it may have different immunopathological characteristics from systemic sclerosis.
Interestingly, the perivascular inflammatory infiltrate from two patients with early stage morphea and one patient with hypertrophic scars showed moderate to high immunopositivity for NICD. Recent evidence suggests the role of immune cells in activation of the Notch signaling pathway in skin fibrosis9,10,14.
Infiltrating T cells prominently expressed the ligand JAG-1 in human systemic sclerosis and fibroblasts stimulated by recruited inflammatory cells that may release NICD, which transforms tumor cells to adopt invasive phenotypes9. Keloid samples with abundant immune cells significantly expressed Notch-1 in fibroblasts, but samples with few immune cells showed minimal to no expression of Notch-1 in fibroblasts14. However, overall, all keloid fibroblasts were immunopositive for NICD, although there was little to no adjacent inflammatory infiltrate in our study. Furthermore, Notch-expressing immune cells were observed in normal control skins in this study. There must be a difference between the immune cell characteristics of normal control skin and of skin of patients with fibroproliferative diseases. Thus, the pro-fibrotic role of immune cells needs to be further investigated.
NICD was constitutively expressed in normal keratinocytes of the epidermis, hair follicles, sebaceous gland endothelial cells, and immune cells. Notch signaling has been suggested to regulate epidermis and hair follicle homeostasis15,16. All specimens in our study exhibited high expression of NICD, especially in the upper epidermal layer15, and Notch-1 was reported to regulate early- and late-stage epidermal differentiation16. In contrast, the normal skin specimens used in the study by Dees et al.9 did not show NICD expression in keratinocytes, endothelial cells, and immune cells. This discrepancy is thought to result from differences in methodologies. Thus, there are conflicting results regarding the effects of Notch-1 on skin carcinogenesis2,17.
The epithelial-to-mesenchymal transition (EMT) is a serial process, which consists of a disassembly of epithelial adherence junctions, morphological changes of epithelial cells to myofibroblasts, degradation of basement membranes, and the migration and invasion of myofibroblasts18. EMT normally occurs during hair follicle development and the wound repair process, but it occurs excessively in the progression of cancer or pathological fibrotic conditions such as multiple sclerosis and keloids6,19. This process leads to increased myofibroblast accumulation and scarring11. Recent evidence has shown that the Notch signaling pathway promotes EMT via interactions with the TGF-β signaling pathway in the kidney tubular epithelium20.
The Notch signaling pathway is known to modulate angiogenesis in normal tissues and tumors15, and the inhibition of Notch signaling showed anti-angiogenic effects in keloids14.
Morphea is considered to be a result of the inflammatory cascade following vascular injury13. Hypoxia, reactive oxygen species, and TGF-β1 are suggested to be profibrotic factors in various fibroproliferative skin diseases such as morpheas and keloids5,21,22. The Notch signaling molecules in dermal fibroblasts are stimulated by hypoxia9,10, reactive oxygen species, or elevated levels of Ha-Ras and Ki-Ras23.
In conclusion, the significant immunoreactivity for the NICD was observed in various fibrotic skin diseases, especially in keloids. In contrast, normal skin keratinocytes, immune cells, and endothelial cells constitutively expressed NICD. Skin fibroblasts only expressed NICD under pathologically fibrotic conditions. These results suggest a pro-fibrotic role of the Notch signaling pathway in human skin fibrosis. Further studies are required to establish the functional role for the NICD at the molecular level in the development and progression skin fibrosis.
Figures and Tables
 | Fig. 1Immunohistochemical analysis of Notch intracellular domain expression in skin samples from fibroproliferative skin disorders and healthy controls. Keloid (A), hypertrophic scar (B), dermatofibroma (C), morphea (D), and normal control (E) (A, C, D, E: ×100; B: ×40). |
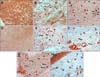 | Fig. 2Immunohistochemical localization of Notch intracellular domain (NICD) in keloid (A, B), hypertrophic scar (C), dermatofibroma (D, E), morhea (F, G), and normal control (H). Keloid fibroblasts showed more intense immunoreactivity than hypertrophic scar and dermatofibroma fibroblasts. Strong to moderate NICD expression is observed in fibroblasts, endothelial cells and infiltrating immune cells in all specimens. Asterisks indicate vessels; arrowheads indicate fibroblasts; arrows indicate infiltrating immune cells (A, C, D, G: ×100; B, E, F, H: ×400). |
 | Fig. 3Immunohistolocalization of Notch intracellular domain in various fibroproliferative skin diseases and normal skin. |
ACKNOWLEDGMENT
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110763) and Clinical Research Laboratory of the Catholic University of Korea, St. Paul's Hospital (2011).
References
1. Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009; 16:633–647.

2. Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007; 28:339–363.

3. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009; 137:216–233.

4. Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007; 134:1243–1251.

5. Kavian N, Servettaz A, Weill B, Batteux F. New insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J. 2012; 6:96–102.

6. Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M, Hara K, Aoki Y, et al. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway. Am J Respir Cell Mol Biol. 2011; 45:136–144.

7. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002; 8:979–986.

8. Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011; 163:141–172.

9. Dees C, Zerr P, Tomcik M, Beyer C, Horn A, Akhmetshina A, et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011; 63:1396–1404.

10. Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011; 70:1304–1310.

11. Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010; 18:139–153.

12. Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004; 23:1155–1165.

13. Beer TW, Lam MH, Heenan PJ. Tumors of fibrous tissue involving the skin. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. 10th ed. Phyiladelphia: Lippincott Williams & Wilkins;2009. p. 969–980.
14. Syed F, Bayat A. Notch signaling pathway in keloid disease: enhanced fibroblast activity in a Jagged-1 peptide-dependent manner in lesional vs. extralesional fibroblasts. Wound Repair Regen. 2012; 20:688–706.

15. Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008; 49:187–194.

16. Lin HY, Kao CH, Lin KM, Kaartinen V, Yang LT. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011; 6:e15842.

17. Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996; 86:531–542.

18. Matsuno Y, Coelho AL, Jarai G, Westwick J, Hogaboam CM. Notch signaling mediates TGF-β1-induced epithelial-mesenchymal transition through the induction of Snai1. Int J Biochem Cell Biol. 2012; 44:776–789.

19. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003; 112:1776–1784.

20. Nyhan KC, Faherty N, Murray G, Cooey LB, Godson C, Crean JK, et al. Jagged/Notch signalling is required for a subset of TGFβ1 responses in human kidney epithelial cells. Biochim Biophys Acta. 2010; 1803:1386–1395.

21. Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008; 105:6392–6397.

22. Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005; 16:667–675.

23. Svegliati S, Cancello R, Sambo P, Luchetti M, Paroncini P, Orlandini G, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005; 280:36474–36482.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download