Abstract
Background
Few studies have compared the efficacy, cosmetic outcomes, and adverse events between 5-aminolevulinic acid photodynamic therapy (ALA-PDT) and methyl aminolevulinate-PDT (MAL-PDT) for actinic keratoses (AKs) in Asian ethnic populations with dark-skin.
Objective
We retrospectively compared the long-term efficacy, recurrence rates, cosmetic outcomes, and safety of ALA-PDT versus MAL-PDT for facial AKs in Koreans.
Methods
A total of 222 facial AKs in 58 patients were included in this study. A total of 153 lesions (29 patients) were treated with 5-ALA, and 69 lesions (29 patients) with MAL. ALA and MAL creams were applied for 6 hours and 3 hours, respectively; the lesions were then illuminated with a halogen lamp at 150 J/cm2 for ALA-PDT and a diode lamp at 37 J/cm2 for MAL-PDT.
Results
The complete response rates of ALA-PDT and MAL-PDT were 56.9% and 50.7%, respectively, with no significant difference at 12 months after treatment. No significant difference in recurrence rates was observed between the 2 PDT modalities at either 6 or 12 months after treatment. There was no significant difference in the cosmetic outcomes between the 2 treatment modalities at 12 months after PDT. However, ALA-PDT caused significantly more painful than MAL-PDT (p=0.005). The adverse events were mild to moderate, transient, and self-limiting for both modalities.
Actinic keratoses (AKs) are common premalignant skin lesions that typically present as single or multiple lesions on sun-exposed areas such as the face and scalp of fair-skinned populations1. It has been estimated that 60% of predisposed persons aged >40 years have had at least 1 AK2. AKs have been estimated to evolve into squamous cell carcinoma in 0.025% to 16% of cases, which is why treatment is recommended3. Many effective treatments for AKs are available, including ablative procedures (surgery, laser ablation, curettage, cryotherapy) and topical treatments (photodynamic therapy [PDT], 5-fluorouracil, 3% diclofenac gel, and 5% imiquimod cream)4. Currently, photodynamic therapy is the first-line therapeutic option for multiple AKs, owing to its efficacy and excellent cosmetic outcomes5.
PDT is based on the activation of photosensitizers, which form cytotoxic oxygen radicals, causing tissue damage and cell death6. The most common photosensitizers used for PDT are 5-aminolevulinic acid (5-ALA) and methyl aminolevulinate (MAL)7. 5-ALA is a hydrophilic molecule that has limited ability to penetrate through cellular membranes and into the interstitial tissue space7,8. MAL is an ALA ester with enhanced lipophilicity, which enables enhanced penetration and a greater target-cell specificity7,9. For these reasons, most dermatologists believe that MAL-PDT may be more effective than ALA-PDT for multiple facial AKs.
To date, numerous studies about the efficacy, cosmetic outcomes, and safety of ALA-PDT or MAL-PDT for multiple AKs have been conducted in Caucasian populations10,11,12,13,14,15,16,17. However, there are insufficient data about the efficacy of either PDT modality for multiple AKs in dark-skinned ethnic groups18,19,20,21,22,23. Furthermore, few studies have evaluated the efficacy of ALA-PDT with a long-term follow-up period in Asian populations23. Previous studies showed that the efficacy of ALA-PDT in Asian patients was similar to that in Caucasian patients18,19,20,23. However, higher light doses, longer irradiation times, and several treatment sessions were required because melanin absorbs the light intended for protoporphyrin IX (PpIX) in dark skin types18,19,20,23. Because there are differences in the long-term efficacy and safety of PDT between Asian and Caucasian populations, data obtained from Caucasians cannot necessarily be applied to the Asian population.
Therefore, we performed this retrospective study to compare the long-term efficacy, recurrence rates, cosmetic outcomes, pain intensity, and safety between ALA-PDT and MAL-PDT for multiple facial AKs in Korean patients.
A total of 58 Korean patients with multiple facial AKs participated in the study after providing written informed consent between November 2007 and December 2011. All patients with 2 to 10 facial AK lesions were included. The diagnosis was based on the results of clinical assessment and histopathological analysis. The exclusion criteria included immunosuppression, porphyria, known allergy to 5-ALA or MAL, pregnancy or lactation, and conditions associated with a risk of poor compliance. Patients who underwent regular ultraviolet radiation therapy or treatment of the face with local therapy (including cryotherapy and curettage) in the previous month, or topical therapy (including imiquimod and 5-fluorouracil) in the previous 3 months were excluded. The study was approved by the institutional review board of the Dong-A University Medical Center, and was performed in accordance with the Declaration of Helsinki (1975).
The 58 patients were divided into 2 groups according to the application of 5-ALA (n=29, 50.0%) or MAL (n=29, 50.0%). The patients used either a 20% 5-ALA cream (Levulan Kerastick; DUSA Pharmaceuticals, Wilmington, MA, USA) or a 16% MAL cream (Metvix; PhotoCure ASA, Oslo, Norway). Before treatment, each AK lesion was photographed, numbered, and graded according to the classification system given by Olsen et al.24. The overall thickness of each lesion was classified into grades 1 to 3, where 1=mild (slightly palpable AK, more easily felt than seen), 2=moderate (moderately thick AK, easily felt), and 3=severe (very thick or obvious AK). Before PDT, excessive loose crusts and debris were gently removed. A 1-mm layer of ALA or MAL cream was applied over the AK lesions, including a 5-mm area of the surrounding normal tissue. Then, the area was covered with a polyurethane film (Tegaderm; 3M, Minneapolis, MN, USA) and aluminum foil to prevent exposure to light. After 3 hours or 6 hours of incubation (ALA, 6 hours; MAL, 3 hours), the occlusive dressing was removed and the cream was washed off with saline. Immediately after this, the ALA-PDT-treated lesion was illuminated with a halogen lamp (Quntzline; Topbulb, East Chicago, IN, USA) placed 30 cm away from the skin surface, with a total light dose of 144 J/cm2 and a light intensity of 120 mW/cm2. The MAL-PDT treatment area was illuminated simultaneously with red light (Aktilite CL 128; Galderma Laboratories, Lausanne, Switzerland) placed 5 cm away from the skin surface for 9 minutes and 20 seconds at 37 J/cm2. Patients wore close-fitting eye shields to protect the eyes during illumination.
All patients attended follow-up evaluations at 1 week, 3 months, 6 months, and 12 months after treatment for response assessment. After a complete response was achieved, all lesions were biopsied at the patients' visit when a recurrence of the lesion was clinically suspected.
The clinical treatment efficacy was measured at 3, 6, and 12 months after PDT. The clinical response was classified as either a complete response (complete disappearance of the lesion) or noncomplete response (incomplete disappearance of the lesion) by using visual examination and palpation. The recurrence rates were evaluated as follows: first, we diagnosed complete-response lesions 3 months after PDT. Subsequently, we reexamined the lesions at 6 and 12 months after PDT to validate the recurrence rates.
The cosmetic outcomes of patients were assessed and graded as excellent (only slight occurrence of redness or change in pigmentation), good (moderate redness or change in pigmentation), fair (slight to moderate scarring, atrophy, or induration), or poor (extensive scarring, atrophy, or induration) in completely cured lesions at 12 months after treatment in both the PDT groups.
Patients were asked to evaluate their pain during lesion illumination and after PDT. Pain was evaluated by using a visual analogue scale (VAS) of 0 to 10, with 0 indicating no pain and 10 representing the worst imaginable pain.
Immediately after PDT and at 1 week after the treatment, short-term adverse events such as erythema, burning sensation, hyperpigmentation, crust formation, pruritus, edema, and bullae were graded as follows: mild (the event was transient and easily tolerated), moderate (the event caused the patient discomfort and interrupted their usual activities), and severe (the event caused considerable interference with the patient's usual activities, and may have been incapacitating or life-threatening). In addition, the number of long-term adverse events, such as the development of new lesions and skin cancer, was assessed at 3, 6, and 12 months after treatment in both the groups.
The treatment efficacy and recurrence rates were tested by using the paired Student t-test. The local adverse events and patient cosmetic outcomes were compared by using contingency tables and the chi-squared test or Fisher exact test. The outcome variable was lesion count, and the predictor variable was time, modeled as a categorical variable. We adjusted for the within-person correlation to account for the correlation in the same patient across different time points. Pain was graded by using the 1-way ANOVA test. The level of statistical significance was set at p<0.05. All statistical analyses were performed by using a software package (SPSS version 15.0; SPSS Inc., Chicago, IL, USA).
The baseline characteristics of both PDT populations, including age, duration of lesions, sex, skin type, overall thickness grade, and number of lesions before the treatment, are summarized in Table 1. The study population consisted of 58 patients, including 15 men and 43 women with a mean age of 69.2±8.8 years (range, 52~87 years). A total of 29 patients with 153 lesions were treated with ALA-PDT, and 29 patients with 69 lesions were treated with MAL-PDT. Both the groups of patients had similar demographic and baseline characteristics. Moderate to severe AKs were observed in 66.7% of patients in the ALA-PDT group and in 91.3% of patients in the MAL-PDT group. The ALA-PDT group included slightly more number of patients with Fitzpatrick skin type III and V and fewer patients with skin type IV than the MAL-PDT group, with no significant difference.
ALA-PDT was similar to MAL-PDT with respect to the complete response rate at 3, 6, and 12 months after treatment, with no significant difference (Fig. 1). At 3 months, the complete response rates were comparable between the ALA-PDT and MAL-PDT groups (68.0% vs. 62.3%; p=0.410). The efficacy at 6 months after PDT was 63.4% for ALA-PDT and 56.5% for MAL-PDT (p=0.441). At 12 months, the complete response rates were similar in the ALA-PDT and MAL-PDT groups (55.0% vs. 45.1%; p=0.395). Both PDT modalities demonstrated significantly superior efficacy at 3 months compared with that at 12 months (p=0.015). For the complete response rates at 3, 6, and 12 months after PDT according to the Olsen grade, a higher efficacy was observed in mild (grade 1) AKs compared with that in severe (grade 3) lesions. At 3, 6, and 12 months after PDT, the Olsen grade-based response rates were similar between both the groups, with no significant difference (Table 2). Histopathological and clinical improvements of the representative moderate to severe cases of AK were observed with both ALA-PDT and MAL-PDT (Fig. 2, 3).
The recurrence rates at 6 months after treatment were 6.7% for ALA-PDT and 9.3% for MAL-PDT, with no significant difference. There was also no significant difference in the recurrence rates between both the modalities at 12 onths after treatment (ALA-PDT, 16.3% vs. MAL-PDT, 18.6%). There was a lower tendency of recurrence in the ALA-PDT group than in the MAL-PDT group at 6 and 12 months after treatment, although this difference was not statistically significant. The lesion recurrence rates were lowest at 6 months, and then significantly increased gradually up to 12 months post-PDT for both modalities (p=0.013; Fig. 4). On the basis of the Olsen grade, mild AKs showed lower recurrence rates than the other subgroups of AKs in both PDTs (data not shown).
The cosmetic outcome after treatment was graded as excellent or good by 89.6% of patients who received ALA-PDT and 96.5% of patients who received MAL-PDT. There was no significant difference in the overall cosmetic outcomes at 12 months after PDT between both the modalities (p=0.557; Fig. 5).
All patients experienced mild to severe pain during lesion illumination. During the illumination procedure, MAL-PDT caused significantly less pain than ALA-PDT (p=0.005), with a median VAS score of 3.4±2.0 and 5.0±2.4, respectively (Fig. 6).
All the patients treated with ALA-PDT (29 of 29; 100%) and MAL-PDT (29 of 29; 100%) reported adverse events at the treatment site. The most common types of reactions were erythema (ALA, 75.8%; MAL, 72.4%), burning sensation (ALA, 79.3%; MAL, 65.5%), hyperpigmentation (ALA, 72.4%; MAL, 65.5%), crust formation (ALA, 69.0%; MAL, 55.2%), pruritus (ALA, 31.0%; MAL, 24.1%), edema (ALA, 20.7%; MAL, 7.0%), and bullae (ALA, 10.3%; MAL, 7.0%) (Table 3). Most adverse events were of mild or moderate grade, and lasted for a short period. All patients continued treatment because the adverse effects were tolerable. At 12 months after treatment, the number of new lesions was not significantly different between both the treatment groups; 12 new lesions occurred in the ALA-PDT group and 11 new lesions occurred in the MAL-PDT group. There was no occurrence of skin cancer in both the PDT groups at 3, 6, and 12 months after treatment.
PDT is the first-line treatment modality for AKs because of its excellent efficacy and cosmetic outcomes24. Some comparative studies of conventional treatment modalities such as cryotherapy10,25, topical 5-fluorouracil26, and MAL-PDT for AKs demonstrated similar efficacy and cosmetic outcomes. Numerous studies have demonstrated the efficacy of ALA-PDT11,12,13 or MAL-PDT10,14,15,16,17 in multiple AKs. In previous studies with ALA-PDT, complete response rates were reported in 73% to 91% of cases11,12,13. Previous studies on MAL-PDT have shown complete response rates of 69% to 92% for AKs on the face and scalp of Caucasian populations10,14,15,16,17. To date, there have been insufficient studies on dark-skinned ethnic groups that have demonstrated the efficacy of ALA-PDT for AKs18,19,20,23. Nakano et al.18 showed that ALA-PDT is useful for the treatment of AKs in Asians, and its efficacy might depend on the lesion size and histopathological severity. However, several treatment sessions (3~6) are needed to achieve a similar efficacy as in Caucasians, and 2 other studies on ALA-PDT reported that its efficacy was 73% to 82%19,23. According to a study performed in Korea, ALA-PDT for AKs on the face and scalp showed a complete response rate of 67% after a single session of ALA-PDT20. Additionally, in that study, the efficacy of ALA-PDT was 92% after 1 to 2 sessions20. Compared with this previous Korean study, a similar efficacy of ALA-PDT was observed in the present study. In addition, few studies have evaluated the efficacy of MAL-PDT for AKs in Koreans21,22. Three months after a single treatment with MAL-PDT, Park et al.21 recorded total clearing of 66% of lesions on the face and chest. Suh et al.22 assessed patients 3 months after their last treatment, and documented a histopathological complete response rate of 86% after 2 sessions of MAL-PDT. Our results showed that 12 weeks after treatment, the overall complete response rate was consistent with the results of that of the previous Korean study reporting a 66% complete response rate after a single session of MAL-PDT.
In this study, we demonstrated that ALA-PDT and MAL-PDT were equally effective in treating multiple AKs (68.0% vs. 62.3%, respectively). The efficacy of both PDT modalities after the single session assessed in our study was slightly lower than that in previous studies (68.0% with ALA-PDT in our study vs. 73%~91%11,12; 62.3% with MAL-PDT in our study vs. 69%~92%10,16). There are several reasons for this result. First, 2 treatment sessions of PDT were more effective than a single treatment session for multiple AK lesions because of increased tissue damage and dysplastic cell death in neoplastic lesions15,16. In our study, decreased efficacy was observed because the number of treatment sessions was lower than that of previous studies10,11,12,13,14,15,16,17,18,19,20,22,23. Second, PDT of mild AK lesions shows higher complete response rates compared with that of moderate and severe AKs10,27, as severe AKs are characterized histopathologically on the basis of considerable overlying hyperkeratosis and parakeratosis, which may disturb ALA and MAL absorption within abnormal cells during the illumination of lesions28. The higher proportion of moderate to severe AKs (ALA-PDT, 66.7%; MAL-PDT, 91.3%) in our study compared with that of the other studies may have influenced the lower efficacy shown in our study10,11,12,13,14,15,16,17,18,19,20,21,22,23. Finally, the facial AK treatment efficacy in Asian patients was similar to that in Caucasian patients; however, 2 to 6 treatment sessions were required18,20,21,22. Additionally, higher light doses and longer irradiation times were required for dark-skinned patients relative to those required for Caucasian patients19. These findings suggest that the efficacy of both PDT modalities is lower in Asian populations than in Caucasian populations. Gerritsen et al.29 reported that ALA-PDT is ineffective for pigmented basal cell carcinoma as a standard treatment because melanin absorbs the photoactivating light intended for PpIX. A previous study reported that melanomas have a poor response to PDT because melanin absorbs light over the entire wavelength region used for PDT (400~750 nm)30. In this study, a single ethnic population (Asian only) consisting only of individuals with dark skin (Fitzpatrick skin types III to V) was evaluated, which may have contributed to the lower complete response rates of PDT. Therefore, the treatment efficacy of ALA-PDT and MAL-PDT in our study was slightly lower than that in Caucasian populations, and repeated treatment is recommended for multiple AKs in Asian populations. Furthermore, pretreatment procedures (including keratolytics, curettage/debulking, tape stripping, microdermabrasion, and laser ablation) for hyperkeratosis is needed because the penetration enhancers may alter the composition of the intercellular lipids and the barrier function of the stratum corneum for AKs in Asian patients29.
A higher complete response rate was observed with MAL than with ALA because the enhanced lipophilicity of MAL increased its penetration and tumor cell specificity7,9. It has been demonstrated that MAL-PDT is more effective than ALA-PDT in multiple facial AKs. In previous studies with ALA for treatment of AK, the efficacy of PDT was 73% to 91% in Caucasians11,12,13, and other studies have reported complete response rates of 73% to 82% in Asian populations18,19. In addition, previous studies have reported the efficacy of MAL-PDT to be 69% to 92% for AK lesions on the face or scalp in Caucasians10,14,15,16,17. These studies demonstrated that PDT has a significant efficacy in AKs, whereas there is no significant difference between ALA and MAL in terms of efficacy. The overall complete response rates between MAL-PDT and ALA-PDT showed no significant difference. MAL-PDT (62.3%) showed a lower tendency toward a complete response than ALA-PDT (68%) because the MAL group had a higher proportion of moderate to severe AKs in our study (ALA, 66.7%; MAL, 91.3%). In this study, the long-term efficacy of AK peaked at 3 months and then decreased gradually. Our results were consistent with those of a previous long-term follow-up study31.
Until now, the time point for maximum porphyrin accumulation because of 5-ALA application is still controversial for AKs32,33. In many clinical studies, the application time of 5-ALA was 3 to 6 hours11,13,18,19,20,23. In a previous study, the total porphyrin levels in basal cell carcinoma and squamous cell carcinoma showed maximum values of 2 to 6 hours32. In addition, the effect of PpIX fluorescence after the application of ALA is dependent on the duration of the application33. Therefore, we performed 5-ALA treatment for 6 hours to achieve high complete response rates.
In previous studies, the recurrence rates at 12 months after ALA-PDT were reported to be 3.5% to 19.0% for AKs of the face and scalp in Caucasians34,35. A study conducted by Nakano et al.18 reported no recurrence both clinically and histologically in Asian patients at 12 months after 3 or 4 sessions of ALA-PDT. Additionally, Dirschka et al.36 reported a 25.4% recurrence rate at 12 months after MAL-PDT. In our study, the tendency of recurrence was lower in the ALA-PDT group than in the MAL-PDT group at 12 months after treatment, with no significant difference (ALA, 16.3%; MAL, 18.6%). At 12 months, the recurrence rates according to the Olsen grade were similar, with no significant difference between both the methods. The larger number of moderate to severe AKs in the MAL-PDT group in our study may have played a role in the higher recurrence rates observed in this group compared with that in the ALA-PDT group. The recurrence rates with both PDT modalities in our study were in agreement with previous studies; however, long-term follow-up studies are needed to accurately evaluate the long-term recurrence rates.
In this study, the cosmetic outcomes of MAL-PDT were better (96.5%) than those of ALA-PDT (89.6%) at 12 months after treatment; however, this difference was not statistically significant. The cosmetic outcomes of ALA-PDT and MAL-PDT were in accordance with those of previous studies10,11,25,37,38. Previous studies also demonstrated that PDT seems to be associated with a better cosmetic outcome than other treatment options including cryotherapy, 5-fluorouracil, and 5% imiquimod cream (up to 90%)10,11,25,26,37,38. Because of the selectivity of MAL for neoplastic cells, optimal destruction of AK is achieved while the normal skin is left intact8.
All patients experienced pain with variable intensity (mild to severe) during the illumination procedure in our study. Our results demonstrated that MAL-PDT (VAS, 3.4±2.0) was significantly less painful than ALA-PDT (VAS, 5.0±2.4) during illumination. These findings were in close accordance with those of several previous studies38,39,40,41. Kasche et al.39 compared the tolerability of MAL and ALA. ALA is thought to be transported by β-amino acids and γ-aminobutyric acid carriers, which suggests a greater uptake in peripheral nerve endings, causing increased pain40. In another previous study, the rate of PpIX photobleaching was used as an indicator of pain intensity and the results showed that the time for pain induction was proportional to the rate of PpIX photobleaching41. The results demonstrated that, in normal skin, ALA induced 3-fold more PpIX than MAL, which might explain the apparent difference in pain scores41. Wiegell et al.42 reported that MAL induced less fluorescence, and was associated with decreased pain during illumination when compared with those for ALA. PDT at low fluorescence rates has been shown to be consistently associated with low pain scores, as low fluorescence rates reduced the rate of tissue hypoxia in a previous study43. Moreover, Steinbauer et al.44 considered the use of ALA as a predictive factor for higher pain levels, in contrast with MAL. More studies are necessary to determine the most appropriate PDT techniques and to elucidate the pathogenesis of PDT-induced pain.
The most common local adverse events in our study were erythema, burning sensation, hyperpigmentation, crust formation, pruritus, edema, and bullae. The profile of local adverse events mainly reflected the expected, local, phototoxic reactions9,16,17. In our study, the symptoms were generally self-limiting, relatively transient, and of mild to moderate intensity. Furthermore, the incidence of adverse events with ALA-PDT was not significantly different from that of MAL-PDT.
Therefore, ALA-PDT and MAL-PDT are both attractive treatment options for facial AKs, with comparable efficacy, recurrence rates, cosmetic outcomes, and adverse events during a long-term follow-up period. However, MAL-PDT is a more appropriate treatment considering the pain intensity observed in our study.
Only a single previous study has directly compared the efficacy and pain severity of ALA-PDT and MAL-PDT in multiple AKs on the scalp45. Moloney and Collins45 showed that the efficacy of MAL-PDT was 71%, whereas that of ALA-PDT was 87%. Both PDT methods resulted in a significant reduction in AK lesions, but they did not significantly differ in terms of efficacy. However, ALA-PDT was more painful than MAL-PDT in extensive scalp AKs45. The previous study showed that both photosensitizers had a comparable efficacy in PDT, although MAL-PDT was less painful than ALA-PDT45. In our study, the efficacy after a single session of both PDTs was slightly lower than that observed in the study by Moloney and Collins45 because of the high proportion of moderate to severe AKs and the dark skin type of the subjects. However, we observed a similar result for the efficacy and pain intensity in both the PDT groups.
To the best of our knowledge, this is the first study that compared the efficacy, recurrence rates, cosmetic outcomes, and safety of ALA-PDT versus MAL-PDT with a long-term follow-up in Asians. However, the results of our study are limited by the small sample size and the single ethnic group (Koreans) composition of subjects, and therefore may not be representative for all patients with AK. Therefore, a multicenter study with a large sample size and including various ethnic populations should be performed to confirm the results of this study.
In conclusion, the long-term efficacy, recurrence rates, cosmetic outcomes, and adverse events of a single MAL-PDT session were similar to those of ALA-PDT. However, the pain severity associated with MAL-PDT was significantly lower than that associated with ALA-PDT. Therefore, we suggest that MAL-PDT may have more advantages than ALA-PDT for the treatment of multiple facial AKs in terms of pain intensity, although both PDT methods have a high complete response rate, good cosmetic outcomes, and less adverse events than other treatment modalities for multiple AKs.
Figures and Tables
 | Fig. 1Complete response rates at 3, 6, and 12 months after methyl aminolevulinate-photodynamic therapy (MAL-PDT) and 5-aminolevulinic acid (ALA)-PDT. *Statistically significant, p<0.05. |
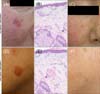 | Fig. 2Clinical and histopathological images of the representative cases treated for Olsen grade 2 (moderate) actinic keratosis with 5-aminolevulinic acid photodynamic therapy (ALA-PDT) (A~C) and methyl aminolevulinate (MAL)-PDT (D~F). (A, D) Clinical images at baseline before treatment with ALA-PDT and MAL-PDT. (B, E) Histopathological images after treatment with both PDT modalities (H&E, ×80). (C, F) Follow-up at 12 months. |
 | Fig. 3Clinical and histopathological images of the representative cases treated for Olsen grade 3 (severe) actinic keratosis with 5-aminolevulinic acid photodynamic therapy (ALA-PDT) (A~C) and methyl aminolevulinate (MAL)-PDT (D~F). (A, D) Clinical images at baseline before treatment with ALA-PDT and MAL-PDT. (B, E) Histopathological images after treatment with both PDT modalities (H&E, ×80). (C, F) Follow-up at 12 months. |
 | Fig. 4Recurrence rates at 6 and 12 months after methyl aminolevulinate photodynamic therapy (MAL-PDT) and 5-aminolevulinic acid (ALA)-PDT. *Statistically significant, p<0.05. |
 | Fig. 5Cosmetic outcomes at 12 months after 5-aminolevulinic acid photodynamic therapy (ALA-PDT) and methyl aminolevulinate (MAL)-PDT. |
 | Fig. 6Pain severity (visual analogue scale [VAS] score) during lesion illumination in the 5-aminolevulinic acid photodynamic therapy (ALA-PDT) and methyl aminolevulinate (MAL)-PDT groups. *Statistically significant, p<0.05. |
References
1. Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000; 42:4–7.

2. Drake LA, Ceilley RI, Cornelison RL, Dobes WL, Dorner W, Goltz RW, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol. 1995; 32:95–98.
4. Sotiriou E, Apalla Z, Maliamani F, Zaparas N, Panagiotidou D, Ioannides D. Intraindividual, right-left comparison of topical 5-aminolevulinic acid photodynamic therapy vs. 5% imiquimod cream for actinic keratoses on the upper extremities. J Eur Acad Dermatol Venereol. 2009; 23:1061–1065.

5. Braathen LR, Szeimies RM, Basset-Seguin N, Bissonnette R, Foley P, Pariser D, et al. International Society for Photodynamic Therapy in Dermatology, 2005. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. J Am Acad Dermatol. 2007; 56:125–143.

6. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992; 14:275–292.
7. Gold MH. Therapeutic and aesthetic uses of photodynamic therapy part five of a five-part series: ALA-PDT and MAL-PDT what makes them different. J Clin Aesthet Dermatol. 2009; 2:44–47.
8. Kloek J, Beijersbergen van Henegouwen GMJ. Prodrugs of 5-aminolevulinic acid for photodynamic therapy. Photochem Photobiol. 1996; 64:994–1000.
9. Fritsch C, Homey B, Stahl W, Lehmann P, Ruzicka T, Sies H. Preferential relative porphyrin enrichment in solar keratoses upon topical application of delta-aminolevulinic acid methylester. Photochem Photobiol. 1998; 68:218–221.

10. Szeimies RM, Karrer S, Radakovic-Fijan S, Tanew A, Calzavara-Pinton PG, Zane C, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002; 47:258–262.

11. Szeimies RM, Karrer S, Sauerwald A, Landthaler M. Photodynamic therapy with topical application of 5-aminolevulinic acid in the treatment of actinic keratoses: an initial clinical study. Dermatology. 1996; 192:246–251.

12. Jeffes EW, McCullough JL, Weinstein GD, Fergin PE, Nelson JS, Shull TF, et al. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch Dermatol. 1997; 133:727–732.

13. Piacquadio DJ, Chen DM, Farber HF, Fowler JF Jr, Glazer SD, Goodman JJ, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004; 140:41–46.
14. Pariser DM, Lowe NJ, Stewart DM, Jarratt MT, Lucky AW, Pariser RJ, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003; 48:227–232.

15. Freeman M, Vinciullo C, Francis D, Spelman L, Nguyen R, Fergin P, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatolog Treat. 2003; 14:99–106.

16. Tarstedt M, Rosdahl I, Berne B, Svanberg K, Wennberg AM. A randomized multicenter study to compare two treatment regimens of topical methyl aminolevulinate (Metvix)-PDT in actinic keratosis of the face and scalp. Acta Derm Venereol. 2005; 85:424–428.

17. Szeimies RM, Matheson RT, Davis SA, Bhatia AC, Frambach Y, Klövekorn W, et al. Topical methyl aminolevulinate photodynamic therapy using red light-emitting diode light for multiple actinic keratoses: a randomized study. Dermatol Surg. 2009; 35:586–592.

18. Nakano A, Tamada Y, Watanabe D, Ishida N, Yamashita N, Kuhara T, et al. A pilot study to assess the efficacy of photodynamic therapy for Japanese patients with actinic keratosis in relation to lesion size and histological severity. Photodermatol Photoimmunol Photomed. 2009; 25:37–40.

19. Itoh Y, Ninomiya Y, Henta T, Tajima S, Ishibashi A. Topical delta-aminolevulinic acid-based photodynamic therapy for Japanese actinic keratoses. J Dermatol. 2000; 27:513–518.

20. Lee JS, Kim YJ, Kang HY, Lee ES, Oh CH, Kim YC. Topical photodynamic therapy for treatment of actinic keratosis using light-emitting diode (LED) device. Korean J Dermatol. 2005; 43:469–474.
21. Park HS, Jin SP, Cho KH. Topical photodynamic therapy with methyl-aminolevulinic acid for the treatment of actinic keratosis. Korean J Dermatol. 2010; 48:837–843.
22. Suh KS, Lee JW, Jeon YS, Kim ST. Efficacy of photodynamic therapy with methyl 5-aminolevulinic acid and red light for actinic keratosis. Korean J Dermatol. 2009; 47:633–640.
23. Hirata Y, Koga S, Fukui N, Yu A, Koshida S, Kosaka Y, et al. 5-Aminolevulinic acid-mediated photodynamic therapy to superficial malignant skin tumors using Super Lizer. J Dermatol. 2011; 38:748–754.

24. Olsen EA, Abernethy ML, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991; 24:738–743.

25. Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I, et al. AKtion Investigators. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006; 155:1029–1036.

26. Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol. 1999; 41:414–418.

27. Togsverd-Bo K, Haak CS, Thaysen-Petersen D, Wulf HC, Anderson RR, Hædersdal M. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012; 166:1262–1269.

28. Smits T, Robles CA, van Erp PE, van de Kerkhof PC, Gerritsen MJ. Correlation between macroscopic fluorescence and protoporphyrin IX content in psoriasis and actinic keratosis following application of aminolevulinic acid. J Invest Dermatol. 2005; 125:833–839.

29. Gerritsen MJ, Smits T, Kleinpenning MM, van de Kerkhof PC, van Erp PE. Pretreatment to enhance protoporphyrin IX accumulation in photodynamic therapy. Dermatology. 2009; 218:193–202.

30. Ma LW, Nielsen KP, Iani V, Moan J. A new method for photodynamic therapy of melanotic melanoma -- effects of depigmentation with violet light photodynamic therapy. J Environ Pathol Toxicol Oncol. 2007; 26:165–172.

31. Szeimies RM, Stockfleth E, Popp G, Borrosch F, Brüning H, Dominicus R, et al. Long-term follow-up of photodynamic therapy with a self-adhesive 5-aminolaevulinic acid patch: 12 months data. Br J Dermatol. 2010; 162:410–414.

32. Fritsch C, Lehmann P, Stahl W, Schulte KW, Blohm E, Lang K, et al. Optimum porphyrin accumulation in epithelial skin tumours and psoriatic lesions after topical application of delta-aminolaevulinic acid. Br J Cancer. 1999; 79:1603–1608.

33. Lesar A, Ferguson J, Moseley H. A time course investigation of the fluorescence induced by topical application of 5-aminolevulinic acid and methyl aminolevulinate on normal human skin. Photodermatol Photoimmunol Photomed. 2009; 25:191–195.

34. Tschen EH, Wong DS, Pariser DM, Dunlap FE, Houlihan A, Ferdon MB. Phase IV ALA-PDT Actinic Keratosis Study Group. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow up. Br J Dermatol. 2006; 155:1262–1269.

35. Sotiriou E, Apalla Z, Chovarda E, Goussi C, Trigoni A, Ioannides D. Single vs. fractionated photodynamic therapy for face and scalp actinic keratoses: a randomized, intraindividual comparison trial with 12-month follow-up. J Eur Acad Dermatol Venereol. 2012; 26:36–40.

36. Dirschka T, Radny P, Dominicus R, Mensing H, Brüning H, Jenne L, et al. AK-CT002 Study Group. AK-CT003 Study Group. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013; 168:825–836.

37. Kaufmann R, Spelman L, Weightman W, Reifenberger J, Szeimies RM, Verhaeghe E, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008; 158:994–999.

38. Serra-Guillen C, Nagore E, Hueso L, Llombart B, Requena C, Sanmartín O, et al. A randomized comparative study of tolerance and satisfaction in the treatment of actinic keratosis of the face and scalp between 5% imiquimod cream and photodynamic therapy with methyl aminolaevulinate. Br J Dermatol. 2011; 164:429–433.

39. Kasche A, Luderschmidt S, Ring J, Hein R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J Drugs Dermatol. 2006; 5:353–356.
40. Rud E, Gederaas O, Høgset A, Berg K. 5-aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem Photobiol. 2000; 71:640–647.

41. Mikolajewska P, Iani V, Juzeniene A, Moan J. Topical aminolaevulinic acid- and aminolaevulinic acid methyl ester-based photodynamic therapy with red and violet light: influence of wavelength on pain and erythema. Br J Dermatol. 2009; 161:1173–1179.

42. Wiegell SR, Stender IM, Na R, Wulf HC. Pain associated with photodynamic therapy using 5-aminolevulinic acid or 5-aminolevulinic acid methylester on tape-stripped normal skin. Arch Dermatol. 2003; 139:1173–1177.

43. Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol. 2008; 158:740–746.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download