Dear Editor:
Hand-foot-mouth disease (HFMD) is a common viral infection that often occurs in small epidemics during the spring or autumn1. Clinically, HFMD is characterized by vesicular, erosive stomatitis, and maculopapular, vesicular lesions on the hands, feet, buttocks and groin. Coxsackievirus A16 (CA16) is the most common cause. Nail matrix arrest can present in several ways: Beau's line, defined as the transverse ridging of the nail plate, and onychomadesis, the complete shedding of the nail from the proximal matrix. Since the first cases reported in 20002, several additional cases of HFMD-associated nail matrix arrest have been reported in the literature1,2,3,4,5,6,7,8,9. To the best of our knowledge, there have been no clinical studies regarding the association between HFMD and nail matrix arrest in Korea. This study was performed by reviewing the retrospective medical records and clinical photographs of 13 patients, who were diagnosed with nail changes, following HFMD. Physical examination was performed, and documented. The extent of nail plate involvement was used to divide the nail change morphology into two subtypes: (i) Beau's lines (Fig. 1A), and (ii) onychomadesis (Fig. 1B). Serologic testing for specific immunoglobulin M antibodies (SRL Laboratories, Tokyo, Japan) to CA6, CA10, CA16, and enterovirus 71 was performed in 3 patients.
The interval from HFMD to the nail changes ranged from 3 to 12 weeks (average 5.92 weeks). The median age was 33 months old (range, 22 months~5 years). Table 1 shows the demographic information and clinical manifestations of the patients. The average number of involved digits was 7.38 (range, 2~14). Fingernails were more commonly involved than toenails; but the most common digit involved was the left great toe (11/13, 84.61%) (Table 1). The palms and soles were most frequently involved (8/13, 61.54%), and the oral cavity was the second most common site involved (7/13, 53.84%). Eight patients reported a history of a fever associated with HFMD, while 5 patients had no history of fever. Patients without a fever history had more affected nails, than patients with a history of a fever. However, there was no statistically significant difference (p=0.35) (independent t-test, PASW Statistics 18.0; IBM Co., Armonk, NY, USA). The average duration of fevers was 3.88 days (range, 1~10), with an average temperature of 39.34℃ (range, 38.5℃~41.0℃). All patients denied any history of major systemic disease or nail trauma, during the 8 weeks prior to the onset of the nail abnormalities. Serological testing was performed in 3 patients. Patients 5 and 6 are twins, while patients 8 and 9 are siblings.
Nail matrix arrest is caused by a temporary arrest in nail plate formation, and is reversible, if the inciting agent is removed1. Although most of the cases are idiopathic, many various conditions-including systemic illness, drugs, fever, periungual dermatitis, trauma, and infection-have been associated with nail matrix arrest1,3. Since the first cases reported in 20002, HFMD has come to be associated with nail matrix arrest, though evidence linking HFMD and nail abnormalities remains unclear. While Clementz and Mancini2 suggested that all patients had been infected with the same viral strain, Bernier et al.4 contended that infection with several viral strains could result in nail matrix arrest. Because patients 5 and 6 were twins, and patients 8 and 9 were siblings, we assumed that both pairs were infected by the same viral strain. Although CA6 was detected in all patients, other viruses were also detected in patients 8 and 11. Like our cases, other reports also describe patients co-infected with several different viral strains3,5,6. Bracho et al.6 reported that CA10 and CB1 were primarily identified as a monoinfection, or co-infection. These authors then concluded that no single serotype is exclusively responsible for post HFMD onychomadesis. Conversely, Wei et al.7 compared the prevalence of onychomadesis between CA6 and non-CA6 infections, during a Taiwanese outbreak of HFMD in 2010, and ultimately concluded that CA6 infections more likely resulted in nail abnormalities, as this subtype was associated with more widespread skin lesions. In our study, patient 8 was found to be infected with four viral strains. Moreover, it is not clear whether these different serotypes infected the patient simultaneously, or sequentially. Similarly, it is also possible that several serotypes were incidentally detected, as serologic testing was performed more than 8 weeks after the onset of HFMD. Therefore, we couldn't identify the responsible virus. The mechanism for post HFMD nail matrix arrest remains unclear. Several theories have been proposed, to describe the underlying pathophysiology. First, nail matrix arrest results from fever occurring during HFMD1. However, in our cases, only 8 patients reported a fever during the duration of the HFMD, and the patients who did not report a fever, had more numbers of affected nails. Additionally, as fevers associated with HFMD are typically low grade, and present only for a few hours, fever seems unlikely as the underlying cause. Second, nail matrix arrest is a result of inflammation or maceration of nail matrix associated finger blisters9. However, most patients in our study did not recall the distributions of cutaneous lesions, with 5 patients denying any skin lesions on the palms and soles. Therefore, we concluded that no relationship exists between nail matrix arrest, and the severity of HFMD. Third, viral replication has been posited as a potential cause. Osterback et al.5 used reverse transcription-polymerase chain reaction (RT-PCR) to detect CA6 in the shed nail fragments. Accordingly, they contend that viral replication may have damaged the nail matrix, thus resulting in temporary nail dystrophy9. As serologic testing was only performed in 3 patients, and RT-PCR was not performed, this association requires further evaluation. Finally, although systemic medications may result in nail matrix arrest, we couldn't confirm accurately which medications were used during the course of HFMD.
From this study, the mechanism of nail matrix arrest remains unclear, as we were unable to elucidate a relationship between viral strain, and nail matrix arrest. However, we found possible associations between nail matrix arrest, and HFMD, as shown in previous studies. Additional studies are needed, to further clarify the virus-associated mechanism of nail matrix arrest following HFMD, and which specific serotype is responsible.
This study was approved and overseen by the Institutional Review Board of Yeouido St. Mary's hospital, the Catholic University of Korea. The clinical trials.gov registration number is SC12RISI0264.
Figures and Tables
Fig. 1
(A) Onychomadesis following hand-foot-mouth disease (HFMD) (patient 8). (B) Beau's lines and onychomadesis on the right index-fingernail following HFMD (patient 3). (C) Beau's lines on the right great toenail following HFMD (patient 1).
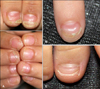
Table 1
Clinical demographics of the 13 enrolled patients with nail changes following HFMD

HFMD: hand-foot-mouth disease, M: male, F: female, NF: not performed, CA: coxackievirus A, L5: left fifth, L4: left fourth, L3: left third, L2: left second, L1: left first, R1: right first, R2: right second, R3: right third, R4: right fourth, R5: right fifth, finger and toe nails, respectively, Rt: right, Lt: left.
ACKNOWLEDGMENT
This work was supported by the Basic Science Research program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012-046972).
References
1. Bettoli V, Zauli S, Toni G, Virgili A. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013; 52:728–730.

2. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000; 17:7–11.

3. Davia JL, Bel PH, Ninet VZ, Bracho MA, González-Candelas F, Salazar A, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011; 28:1–5.

4. Bernier V, Labrèze C, Bury F, Taïeb A. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001; 160:649–651.

5. Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009; 15:1485–1488.

6. Bracho MA, González-Candelas F, Valero A, Córdoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011; 17:2223–2231.

7. Wei SH, Huang YP, Liu MC, Tsou TP, Lin HC, Lin TL, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011; 11:346.

8. Haneke E. Onychomadesis and hand, foot and mouth disease--is there a connection? Euro Surveill. 2010; 15:pil: 19664.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download