Dear Editor:
Leuprorelin acetate is a synthetic analogue of the naturally occurring luteinizing hormone releasing hormone (LHRH), and is produced in 1- and 3-month (3.75 mg and 11.25 mg, respectively) depot preparations, typically administered intramuscularly or subcutaneously, in the upper arm or abdomen1. To date, several cases of leuprorelin acetate granuloma have been reported in the Japanese and European literature1,2, though not in Korean dermatologic journals. Herein, we report a case of a foreign body granuloma, resulting from leuprorelin acetate injections.
A 44-year-old woman presented to our clinic, with three pruritic nodules on the left upper arm. Four months prior, the patient had been treated for endometriosis with leuprolide (LEUPLIN® DPS injection 11.25 mg; Takeda, Osaka, Japan), via two subcutaneous injections in the left upper arm. Three months later, three discrete nodules developed on and near the injection sites. Clinical examination revealed three, 2 to 3 cm-sized, firm, erythematous nodules on the left upper arm (Fig. 1). A 3-mm punch biopsy was performed, under the differential diagnosis of bacterial, fungal, or mycobacterial infection, traumatic panniculitis, or injection site granuloma. Pathologic findings revealed a marked degeneration of adipose tissue, and an inflammatory cell infiltrate, mainly composed of lymphohistiocytes. Foreign body giant cells with translucent intracytoplasmic vacuoles were also noted (Fig. 2), and Ziehl-Neelsen, Gram, and periodic acid-Schiff stains were all negative. The patient was treated with intralesional triamcinolone injections (2.5 mg/ml). On follow-up 1 month later, the size of the lesion was noted to have decreased.
LHRH analogues are widely used to treat sex-hormone-responsive diseases2. Currently, two LHRH analogues are available: leuprorelin acetate (LEUPLIN®; Takeda), and goserelin acetate (Zoladex®; AstraZeneca, Cheshire, U.K.)3. In these depot preparations, LHRH is coupled to biodegradable microcapsules, composed of lactic/glycolic acid polymers1. As LHRH has lipolytic activity in vitro, the degenerated lipid granules may induce foreign body reactions4. Moreover, lactic/glycolic acid polymers remain in tissues for several months, likely further contributing to granulomatous inflammation1.
Though leuprorelin acetate granulomas can in some cases be painful, most are asymptomatic. Firm, subcutaneous nodules or sterile abscesses can develop1. Histopathologic analysis of such lesions reveal multi-nucleated giant cells, containing round, translucent microspheres and granulomas, with secondary foreign body reactions4.
In the case described here, leuprorelin acetate was twice injected subcutaneously in the upper arm, with erythematous nodules developing at the injection site, three months later. The histologic features were identical to those of previously reported leuprorelin acetate granulomas.
Leuprorelin acetate granulomas are rare in Western countries, though many cases have been reported in Japan, with this discrepancy explained by the difference in injection methods3. In Western countries, leuprorelin acetate is administered intramuscularly, while in Korea as well as in Japan, it is generally administered subcutaneously3.
In one study by Shiota et al.3, the incidence rate of leuprorelin acetate granuloma was 4.2% (5 out of 118 cases), with all cases associated with 11.25 mg leuprorelin acetate depot injections. All five patients with granulomas were transitioned to treatment with goserelin acetate, with recurrence only occurring in one individual. Based on these findings, goserelin acetate would seem to have a lower risk of inducing granuloma formation, than leuprorelin acetate. Therefore, switching the patient to intramuscular goserelin acetate is recommended.
Figures and Tables
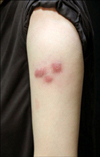 | Fig. 1Three, pruritic, 2~3-cm, erythematous nodules at the site of a previous leuprorelin injection on the left upper arm. |
 | Fig. 2Histopathology of skin lesions. (A) An acanthotic epidermis with a marked degeneration of adipocytes, and an intense inflammatory cell infiltrate, composed of lymphocytes and neutrophils. (B) Foreign-body-type giant cells with translucent intracytoplasmic vacuoles (arrows) (H&E; Inset: ×100, A: ×40, B: ×400). |
References
1. Yasukawa K, Sawamura D, Sugawara H, Kato N. Leuprorelin acetate granulomas: case reports and review of the literature. Br J Dermatol. 2005; 152:1045–1047.

2. Ouchi T, Koyama T, Miyata N, Sugiura M. Granuloma caused by subcutaneous injection of leuprorelin acetate product: case report and histopathological findings. J Dermatol. 2006; 33:719–721.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download