Abstract
Non-tubecrulosis mycobacterium infections were increasingly reported either pulmonary or extrapulmonary in the past decades. In Taiwan, we noticed several reports about the soft tissue infections caused by rapid growing mycobacterium such as Mycobacterium abscessus, Mycobacterium chelonae, on newspaper, magazines, or the multimedia. Most of them occurred after a plastic surgery, and medical or non-medical procedures. Here, we reported two cases of these infections following medical procedures. We also discussed common features and the clinical course of the disease, the characteristics of the infected site, and the treatment strategy. The literatures were also reviewed, and the necessity of the treatment guidelines was discussed.
The rapid growing non-tuberculous mycobacterium (NTM) have increasingly been reported during recent decades, with the trend of the attribution to surgical procedures and immunocompromised patients1,2,3,4,5. The common clinical pathogens of rapid growing mycobacterium (RGM) group include the Mycobacterium abscessus, M. chelonae, and M. fortuitum. These organisms are present in or found extremely in the water, the soil, or the nosocomial sources. Several conditions such as mesotherapy, non-sterile venopuncture, acupuncture, or even tattoo, have been reported to cause the infections of these organisms1,3,4,5,6,7. Due to a gradually increasing incidence of the infections of this group8,9, we reported 2 cases of these types of infections, which were characterized as medical procedures associated infections, and reviewed other literatures about these conditions.
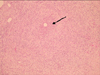
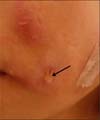
A 45-year-old female without any known medical conditions noticed a painful swelling of both her cheeks after a surgical procedure of auto-fat transplantation from the thigh to both of her cheeks 3 months ago. Multiple erythematous nodules have been found since 2 months ago (Fig. 1). Two weeks ago, a pus discharge from the nodule was noticed. The color of the pus was light yellowish while sticky in character (Fig. 2). She visited our infection out-patient-department (OPD) where the common pus culture was done but showed no growth. The lesions were not improved even after an oral dicloxacillin use. The culture was repeated and the mycobacterial culture also was done on the 2nd visit a week later. Throughout the entire course of the disease, there was no fever or chills. Due to the prolonged time of the course of the disease, she was admitted for an intravenous antibiotic therapy. The previous mycobacterium culture showed Mycobacterium spp. isolation without recognition of the species on the 5th day of the culture. After the admission, an intravenous amoxicillin-clavulanate was given initially. However, the mycobacterial culture of pus reported as M. abscessus on the 2nd day at the hospital (the 18th day since the collection of the mycobacterium culture). The antibiotic was then shifted to intravenous cefmetazole, amikacin in combination with oral clarithromycin. The lesions showed gradual improvement after the antibiotic treatment. The patient was discharged on the 10th day and continued to receive oral clarithromycin at infection OPD.
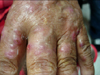
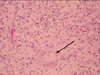
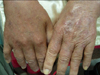
A 79-year-old female who was underlined with type II diabetes mellitus with stage III nephropathy, hypertension, bullous pemphigoid under steroid therapy, old cerebrovascular accident, and coronary artery disease status post percutaneous transluminal coronary angioplasty. She had a history of previous admission to the neurosurgery ward due to 9th thoracic spine osteomyelitis 4 months ago. Nosocomial pneumonia and urinary tract infection also occurred during the admission. Near the end of the hospital stay, the patient began to notice multiple painful erythematous nodules over both of her hands (Fig. 3). After the discharge, she went to our dermatology OPD for help and a local treatment with incision and drainage was performed. A pus formation was noted with sticky character and yellowish color. Due to the non-growth of common bacterial culture, the skin biopsy was done on the 2nd visit. An oral amoxicillin-clavulanate was also prescribed. However, due to no visible improvement, the patient was referred to the infection OPD, and mycobacterial culture was sent due to the previous pathological report showing a granulomatous inflammation (Fig. 4, 5). The oral antibiotic was also changed to dicoxacillin. The mycobacterium culture showed Mycobacterium spp. isolation without recognition of the species on the 7th day. However, the patient was admitted to nephrology due to an episode of urosepsis and an acute renal failure two weeks later. The previous mycobacterial culture done at OPD finally reported a M. chelonae on the 15th day of the culture. An infection specialist was consulted and intravenous imipenem, amikacin, and oral clarithromycin were given. The intravenous antibiotic was discontinued 7 days later but the patient continued to receive oral clarithromycin. The lesion showed marked improvement after one month oftherapy (Fig. 6).
In recent decades, the incidence of soft tissue infection caused by NTM gradually increased7,8,9. Of the extrapulmonary diseases caused by NTM, skin and soft-tissue infection was the most common. Bodle et al.8 declared that the skin and soft tissue were the second most common sites/areas of disease and occurred in 21 (18%) patients of a 256-person-study in New York. Another study by Henry et al.9 in Great Britain also disclosed a notable increased incidence (17%) of skin infections caused by NTM. The RGM group includes the M. fortuitum group, the M. smegmatis group and the M. chelonae-abscessus group. The latter includes M. chelonae, M. immunogenum, M. abscessus, M. bolletii and M. massiliense species10,11. As its name suggests, this group of organisms showed a rapid growth over the culture medium, mostly isolated within seven to ten days. Numerous case reports, case series, or even original studies pointed to the relationship with the medical or non-medical procedures1,3,4,5,6,7. Non-sterile venopuncture, acupuncture, or even tattooing had been reported in South America, Southeastern Asia, or even Europe. The pathogenic potential of NTM has been recognized since the beginning of the last century. Many large series studies have reported that NTM can be classified as pulmonary and extrapulmonary diseases. One epidemiological study in Taiwan showed that the average incidence of NTM skin and soft tissue infections was 1.39 per 100,000 patients between 1997 to 2008, with a decreasing trend in the cases involving immunosupressed patients12. In Taiwan, M. abscessus was found more often in the infection of the localized skin lesion with a higher risk of deep tissue involvement than M. chelonae and other group of NTM such as M. avium complex12.
The most common clinical presentation of RGM infection in patients with defects in their cellular immunity or who have been receiving glucocorticoid therapy is disseminated cutaneous involvement. Immunocompetent hosts can also develop localized cutaneous infection after surgical or traumatic wounds, from contaminated injections, or after a body piercing. Cutaneous lesions are cellulitic or nodular; they are typically erythematous, indurated, and tender and may progress to ulceration and purulent drainage. Proximal sporotrichoid spread, such as our 2nd case, has also been reported. This type of infection tends to occurr in hosts with immunosuppressed status such as patients who had a chronic steroid use, immunosuppressive medications, or such comorbidities as diabetes mellitus, malignant neoplasms, chronic renal or hepatic diseases, rheumatic disease, or acquired immunodeficiency syndrome.
In our cases, the first patient denied any systemic disease and the laboratory data surveyed during the hospital stay showed no abnormality. The normal immunity condition was impressed with this type of infection, as other reported cases associated with plastic surgery1,3,4,5. The second patient has the underlying diseases of type II diabetes mellitus with stage III nephropathy, cerebrovascular accident, coronary artery disease, and long term steroid therapy. The immunocompromised condition was obvious. The source of the RGM was suspected due to the frequent venopuncture during the previous long-term hospital stay. These two cases demonstrated that not only the immunocompromised patients but also the immunocompetent patients could receive the infection of these organisms during various types of medical procedures. The isolation time of the mycobacterial culture in these two cases were five and eight days respectively, compatible with other literature reports.
The treatment of this type of infection showed a poorly established evidence. RGM are resistant to most antituberculous drugs. The regimens for the NTM species infection was suggested to be based on in vitro susceptibility. M. abscessus and M. chelonae are usually susceptible to clarithromycin and amikacin; they are also susceptible to cefoxitin and imipenem. Other effective agents may include doxycycline and fluoroquinolones.
For RGM infections in Taiwan, clarithromycin and fluoroquinolones were the most prescribed drugs12. Forty-eight percent of these patients received clarithromycin and 30% of them received fluoroquinolones. Amikacin and imipenem were given to 22% of the patients with RGM skin and soft tissue infections. The cure rates were reported as 74.2% (M. abscessus) and 92.3% (M. chelonae) respectively12. In two case reports, the recommended antibiotic was clarithromycin if susceptible to the pathogen and could be used alone, especially when the infection is superficial or localized in immunocompetent patients1,7. However, the in vivo efficacy against the mycobacterium is not completely reflected in vitro sensitivity. The resistance to clarithromycin was also reported in several literatures/reports. One of them showed infection from M. fortuitum acquired through acupuncture6. Another preliminary report in Columbia also declared acquired resistance to clarithromycin4. An empiric treatment with other antibiotic was indicated due to the delay in getting the in vitro susceptibility result. A combination therapy with other antibiotics was also recommended due to the reports of resistance to various drugs, as shown in above examples. The treatment course should be maintained for at least four to six months. So far, a systemic antibiotic treatment remains the preferred and recommended strategy other than a topical treatment or a surgical intervention.
Surgical intervention is an alternative option for the treatment of the RGM cutaneous infections. It remains an important adjunctive tool in treating these kinds of infections. In one series, patients with single lesion were far more likely to undergo a surgical debridement than those with multiple lesions (76% vs. 40%)2. An extensive surgical debridement for widespread infections may be technically challenging; however, patients with multiple lesions are more likely to be immunosuppressed and may be less likely to be cured of their infection with antimicrobial agents alone. A combination of medical and surgical therapy is likely to produce optimal results in severe cases. In one report of outbreaks of sternal wound infections due to M. abscessus, approximately one third of the patients died of uncontrolled infections, suggesting that an aggressive therapy may be indicated for deep tissue infections2. No study specifically addressed the issue of the reduction of immunosuppressive agents during the treatment, but it is likely that this may be beneficial, if tolerable by the patient relative to his or her underlying comorbidities. In Taiwan, the rate of surgical intervention only in the NTM infected patients were 12.7% between 1997 to 2004, and 14% between 2005 to 200812. More patients received systemic antibiotics therapy combined with surgical intervention, and the rates were 47.6% between 1997 to 2004 and 56% between 2005 to 200812. The data revealed that a surgical intervention may play an important role in treating this type of infection. However, there was no definite statistical analysis concerning the result and the prognosis following the surgical intervention, whether treated alone or combined with systemic antibiotics. In conclusion, skin and soft tissue infections with RGM in recent decades showed a trend of increase either in incidence or in population. The RGM infected skin and soft tissue following medical or surgical procedures, conventional strategy, or trauma, had poor response to the traditional antibiotics and the common bacterial culture over the infected tissue. In this study, we detailed our experiences and emphasized the clues to the diagnosis in the following way: a rapid recognition of the character of the infection site with the cellulitic or nodular appearance, and ordering mycobacterium culture, especially when the common bacterial culture showed no growth. According to the suggested issues above, we believe that the correct treatment strategy will be achieved by each infection expert or dermatologist. However, a more definite/appropriate treatment guideline is also needed by doctors when facing this kind of clinical conditions as we long for its application in the near future.
Figures and Tables
 | Fig. 1Frontal view of patient's face showed multiple erythematous lesions (arrows) over bilateral cheeks. |
 | Fig. 4Photomicrograph shows histopathological findings from dorsum of hand of the patient. A granulomatous lesion with central abscess formation (arrow) and lymphohistiocytic and multinucleated giant cells infiltration were noted (H&E, ×100). |
References
1. Wongkitisophon P, Rattanakaemakorn P, Tanrattanakorn S, Vachiramon V. Cutaneous mycobacterium abscessus infection associated with mesotherapy injection. Case Rep Dermatol. 2011; 3:37–41.

2. Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006; 142:1287–1292.
3. Toy BR, Frank PJ. Outbreak of Mycobacterium abscessus infection after soft tissue augmentation. Dermatol Surg. 2003; 29:971–973.

4. Sañudo A, Vallejo F, Sierra M, Hoyos JG, Yepes S, Wolff JC, et al. Nontuberculous mycobacteria infection after mesotherapy: preliminary report of 15 cases. Int J Dermatol. 2007; 46:649–653.

5. Garcia-Navarro X, Barnadas MA, Dalmau J, Coll P, Gurguí M, Alomar A. Mycobacterium abscessus infection secondary to mesotherapy. Clin Exp Dermatol. 2008; 33:658–659.
6. Guevara-Patiño A, Sandoval de Mora M, Farreras A, Rivera-Olivero I, Fermin D, de Waard JH. Soft tissue infection due to Mycobacterium fortuitum following acupuncture: a case report and review of the literature. J Infect Dev Ctries. 2010; 4:521–525.

7. Bechara C, Macheras E, Heym B, Pages A, Auffret N. Mycobacterium abscessus skin infection after tattooing: first case report and review of the literature. Dermatology. 2010; 221:1–4.

8. Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008; 14:390–396.

9. Henry MT, Inamdar L, O'Riordain D, Schweiger M, Watson JP. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. Eur Respir J. 2004; 23:741–746.

10. Wilson RW, Steingrube VA, Böttger EC, Springer B, Brown-Elliott BA, Vincent V, et al. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int J Syst Evol Microbiol. 2001; 51:1751–1764.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download