Abstract
Background
Balneotherapy, although not a well-established dermatological treatment, is thought to have therapeutic properties for psoriasis and is used as an alternative treatment modality throughout the world.
Objective
To evaluate the mechanism underlying the therapeutic immunologic effects of thermomineral water.
Methods
A murine model of imiquimod-induced psoriasis-like skin inflammation was used for evaluating the therapeutic effects of balneotherapy with Hae-Un-Dae hot spring mineral water. The clinical improvements were evaluated by a dermatologist. Lesional cytokines, including interleukin (IL)-17A, IL-23, and IL-22, were quantitatively measured by real-time reverse transcriptase polymerase chain reaction. Serum levels of interferon-γ, IL-4, IL-5, and IL-17A were measured by enzyme-linked immunosorbent assay. T cell proportions in the spleen were evaluated by flow cytometry, and histopathological evaluation of the skin was also performed.
Results
The mineral water balneotherapy group showed faster improvement in skin erythema and scales than the distilled water bathing group. A substantial reduction was observed in the lesional mRNA levels of IL-17A and IL-23 in the mineral water group. Serum levels of IL-4 and IL-5 were significantly decreased in the mineral water group but not in the distilled water group. Normalized T cell proportions were observed after bathing.
Conclusion
Balneotherapy showed immunomodulatory effects in a psoriasis-like murine model. Balneotherapy suppressed lesional IL-23 and IL-17A, which are important cytokines in the pathogenesis of psoriasis. These results suggest that balneotherapy can be used as an effective and safe treatment for psoriasis.
Balneotherapy refers to immersion in baths or pools of thermomineral water. Although balneotherapy is not accepted as a well-established treatment, it appears to have therapeutic potential for various diseases. Balneotherapy has been frequently used to treat psoriasis1. Psoriasis has a strong genetic basis, and treatment for psoriasis is aimed at inducing and maintaining remission2. Therefore, the safety of long-term therapy is very important in the treatment of psoriasis. Conventional pharmacologic treatments, including methotrexate, acitretin, cyclosporine, and topical corticosteroids, can be used for limited durations due to their cumulative toxicity and potential side effects.
Many patients with psoriasis request alternative therapies and perceive them as helpful3. One of the promising alternative therapies for psoriasis is balneotherapy. This treatment offers a natural, multifactorial, complementary, and nontoxic alternative to the traditional pharmacologic treatments of psoriasis.
The effects of using various mineral-rich thermal water for treatment of psoriasis have been studied. A majority of clinical studies have used hypertonic Dead Sea water combined with phototherapy1,4,5,6. Comano thermal water, which is characterized as an oligometallic and hypotonic thermal water, has also been evaluated for its effectiveness in treating psoriasis7,8. Balneotherapy with hypotonic mineral water has been shown to improve psoriasis without additional treatment modalities. Balneotherapy has chemical, thermal, mechanical, and immunomodulatory effects. The chemical components of this thermal water, including sulfur, magnesium, and selenium, are thought to have beneficial effects on the skin. Heat may also have an anti-inflammatory effect9. Thermal stimulation causes vasodilatation, enhanced blood circulation, and decreased blood pressure. The moisturizing effect of bathing on the stratum cornea and the desquamation of dead keratinocytes can be beneficial to the skin. In addition, the psychological effects of balneotherapy cannot be overlooked. Despite the benefits of balneotherapy, its mechanism of action is not well established.
The murine imiquimod-induced psoriasis-like model has been widely used for psoriasis study10. Imiquimod application induces epidermal expression of interleukin (IL)-23 and IL-17 as well as an increase in splenic Th17 cells that play a pivotal role in the pathogenesis of psoriasis10. In the present study, the effects of mineral water versus distilled water were evaluated using the imiquimod-induced psoriasis-like murine model. The skin and serum levels of inflammatory cytokines were measured. T cell proportions in the spleen were compared between mice treated with mineral water balneotherapy and distilled water bathing. Histological evaluations of the skin were also performed.
The temperature, pH, electrical conductivity, and alkalinity of the Hae-Un-Dae hot spring water were measured in situ. Collected water samples were filtered with 0.45-µm cellulose membranes and stored in polyethylene bottles. Samples for cation analysis were acidified to a pH of <2 by adding a few drops of ultra-pure nitric acid. The alkalinity was measured in the field using an acid-neutralizing titration technique and then converted to the equivalent HCO3- and CO32- concentrations. Water samples were analyzed for Na+, K+, Ca+, Mg2+, SiO2, and total iron concentrations using induced coupled plasma-atomic emission spectrometry (Perkin Elmer OPTIMA 3000XL; Meinhard, Golden, CO, USA) and atomic adsorption spectrometry (Perkin Elmer Analyst100, Meinhard). Anions such as Cl-, SO42-, PO43-, and F- were analyzed by ion chromatography (Dionex 120; Dionex, Sunnyvale, CA, USA). Quality controls included blank samples and duplicate or triplicate subsamples and standard materials.
Twelve C57BL/6 mice (age, 8 weeks) were obtained from the Ewha Medical Research Institute of Ewha Medical School. Mice were housed and bred under conventional conditions at the animal laboratory of the Ewha Medical School. All animal procedures were approved by the Ethics of Animal Care and Use Committee of Ewha Womans University School of Medicine (ESM 11-0184) and conformed to international standards.
A daily topical dose of 4.15 mg of commercially available imiquimod cream (5% Aldara®, 83g; Dong-A Pharmaceuticals, Seoul, Korea) was applied to the shaved back of nine mice for 5 consecutive days. Three control mice were treated with only a vehicle cream (Vaseline Lanette cream; Fagron, Rotterdam, Netherlands) as the negative control group. Immediately after induction of psoriasis-like skin lesions, three of the nine mice were sacrificed (positive control group) and the other six mice underwent bathing with either distilled water or Hae-Un-Dae hot spring mineral water for 5 minutes a day for 2 weeks. The water was maintained at a constant temperature of 38℃ during the bath.
Clinical photos of the backs of mice were taken at day 1, 4, 7, 10, and 13. Assessment of the severity of skin erythema and scales was performed by a blinded analysis using the clinical photos. The severity of skin erythema and scales were scored independently from 0 to 3 as follows: 0, none; 1, slight; 2, moderate; 3, marked.
A day after the final bath, mice were sacrificed, and the dorsal skin was excised and divided into two specimens, one for histopathological evaluation and the other for mRNA extraction. Total mRNA was extracted from the back skin using TRIzol (Invitrogen, Carlsbad, CA, USA) followed by isopropanol precipitation and a 70% ethanol wash. The RNA pellets were dissolved in diethylpyrocarbonate-treated water. The RNA was quantified and purity evaluated by the 260 : 280 optical density ratio with a target ratio >1.8. Using 1 µg of total RNA template, cDNA was prepared using a reverse transcription system (Promega, Madison, WI, USA) and oligo (dT) and random hexamer primers. Reaction mixtures were amplified with the SYBR® Premix Ex Taq™ (Tli RNaseH Plus) kit (Takara, Shiga, Japan) using a iQ5 real-time polymerase chain reaction (PCR) detection system (Bio-Rad, Hercules, CA, USA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene was used as an internal control. Sequences for PCR primers and reference numbers for probes (Universal Probe Library; Roche Applied Science) are as follows: IL-17A, forward primer, 5'-TTT TCA GCA AGG AAT GTG GA, reverse primer, 5'-TTC ATT GTG GAG GGC AGA C, probe no. 34; IL-22, forward primer, 5'-TTT CCT GAC CAA ACT CAG CA, reverse primer, 5'-CTG GAT GTT CTG GTC GTC AC, probe no. 17; IL-23, forward primer, 5'-CAC CTC CCT ACT AGG ACT CAG C, reverse primer, 5'-TGG GCA TCT GTT GGG TCT, probe no. 25; and GAPDH, forward primer, 5'-TCC ACT GGC GTC TTC AC, reverse primer, 5'-GGC AGA GAT GAT GAC CCT TTT, probe no. 9. Cytokines and GAPDH levels were calculated relative to a standard sample, and cytokine mRNA levels were normalized to GAPDH.
Sampling of blood was performed the day after the final bathing and serum was stored at -70℃ until analysis. Levels of interferon (IFN)-γ, IL-4, IL-5, and IL-17A were measured by enzyme-linked immunosorbent assay (IFN-γ, 430804, BioLegend, San Diego, CA, USA; IL-4, 555232, BD Bioscience, San Diego, CA, USA; IL-5, 555236, BD Bioscience; and IL-17, 432504, BioLegend) according to the manufacturer's instructions.
Spleen samples were minced through a 70-µm mesh to obtain single cell suspensions. Cells were washed twice, and 1×104 cells per staining were fluorescently labeled by incubation for 10 minutes at room temperature with the following monoclonal antibodies diluted in 0.5% fetal bovine serum and phosphate buffered saline (PBS, pH 7.4). To analyze the Th1 cells, spleen cells were stained with phycoerythrin (PE) anti-mouse CD4 (100407, Bio-Legend). The cells were then fixed in 200 µl of 4% paraformaldehyde in PBS and permeabilized. Subsequently, cells were stained with PE anti-mouse IFN-γ (505807, BioLegend). For the analysis of Th2 cells, spleen cells were stained with PerCP anti-mouse CD4 (100431, BioLegend) followed by intracellular staining with allophycocyanin (APC) anti-mouse IL-4 (504105, BioLegend) and PE anti-mouse IL-9 (514103. BioLegend). To stain Th17 cells, spleen cells were fixed, permeabilized, and then stained with PE anti-mouse IL-17A (506903, Bio-Legend). For Foxp3+ regulatory T cells, spleen cells were stained with Alexa Fluor 488 anti-mouse/rat/human Foxp3 (320011, BioLegend). Data were acquired on a FACS-Calibur system (BD Bioscience) and analyzed using Cell-Quest software (BD Bioscience).
The dorsal skin was excised and divided into two specimens, one for histopathological evaluation and the other for mRNA extraction. For histological evaluation, skin samples were fixed with 10% paraformaldehyde and embedded in paraffin. Paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated in a graded series of alcohol solution. Slides were stained with standard hematoxylin (Sigma-Aldrich Chemical Co., St Louis, MO, USA) and eosin (Sigma-Aldrich Chemical Co.). The thickness of the epidermis was examined under an optical microscope (Olympus, AX70, TR-62A02; Olympus Co., Tokyo, Japan).
All data are expressed as the mean±standard deviation. One-way analysis of variance followed by Tukey's multiple comparison tests were used for statistical analyses. All analyses were performed using Statistical Analysis Software version 9.1 (SAS Institute, Cary, NC, USA). Differences were considered statistically significant at p<0.05.
Hae-Un-Dae hot spring mineral water was classified as water with high mineral content based on the total dissolved solids (3,788±741.9 mg/L). It had a pH of 7.6±0.1, which is lower than the average pH of most Korean mineral waters (pH=8.6)11. The electrical conductivity of Hae-Un-Dae mineral water was high (8,980±1,170 µS/cm), indicating a high dissolved ion content. The most common cation present was Na+, followed by Ca2+, K+, and Mg2+. The predominant anion was Cl-, followed by SO42- and HCO3-. A high concentration of SO42- (192.9±23.4 mg/L) was observed when compared to the median level of SO42- (12.3 mg/L) present in the thermal groundwater of South Korea11. The total sulfur content of Hae-Un-Dae mineral water was also relatively high at 77.3 mg/L. Total NO3- content indicates pollution originating from the ground, and NO3- was not detected. In addition, F-, which is abundant in most groundwater of South Korea, was also not detected (Table 1).
Skin inflammation induced by imiquimod presented with skin erythema and scales. Clinical photos of the mice were taken at days 1, 4, 7, 10, and 13 of bathing, and the severity of skin erythema and scales were assessed by an independent dermatologist (Fig. 1). The mineral water balneotherapy group showed increased improvement in skin erythema and scales as compared with the distilled water bath group at days 4 and 7; however, the results were not statistically significant. At day 10, skin recovery was clinically recognized in both bathing groups (Fig. 2). The mineral water balneotherapy groupshowed a tendency towards faster hair growth than the distilled water bath group.
After 5 days of imiquimod application, levels of IL-17A, IL-22, and IL-23 mRNA increased in skin lesions (1.9±0.4 fold increase in IL-17A, 2.0±0.6 fold increase in IL-22, and 2.1±0.3 fold increase in IL-23 over the negative controls treated with Vaseline). The level of IL-17A in the distilled water group (2.0±0.4 fold increase compared with the Vaseline group) was similar to the imiquimod group (positive control) without bath. However, the level of IL-17A in the mineral water group was only 1.6±0.4 fold higher than the Vaseline group. Similar to IL-17A, the level of IL-23 in the mineral water group was only 1.6±0.6 fold higher than the Vaseline group. However, this reduction in IL-23 expression was not observed in the distilled water group (2.3±0.1 fold higher than the Vaseline group). A marked decline was observed in the level of IL-22 in both the distilled water (0.4±0.2 fold) and mineral water (0.2±0.1 fold) treatment groups (Fig. 3).
Serum levels of IFN-γ were not significantly different between imiquimod-induced mice and Vaseline-treated control mice (304.3±25.7 pg/ml and 278.4±14.4 pg/ml, respectively). Levels of IFN-γ in the mineral water group (268.5±19.5 pg/ml) were slightly lower than those in the distilled water group (292.8±0.4 pg/ml); however, this difference was not statistically significant (p=0.198; Fig. 4A). A statistically significant difference was observed in the serum levels of IL-4 between the imiquimod-treated group (positive control, 116.0±1.7 pg/ml) and the Vaseline-treated group (negative controls, 102.7±3.4 pg/ml). Compared with the positive control, IL-4 levels were significantly reduced in the mineral water group (p=0.011; 99.2±1.1 pg/ml) but not in the distilled water group (109.4±7.6 pg/ml, p=0.454; Fig. 4B). Similar findings were observed for serum levels of IL-5. Compared with the positive control (122.7±20.8 pg/ml), a significant difference was observed in the IL-5 levels of the negative control (49.6±14.8 pg/ml, p=0.038) and the mineral water group (54.9±3.3 pg/ml, p=0.043), but not the distilled water group (69.6±17.8 pg/ml) (p=0.20; Fig. 4C). Levels of IL-17A (405.6±44.9 pg/ml, 305.5±9.0 pg/ml, 323.2±17.1 pg/ml, and 332.2±23.1 pg/ml in the positive control, distilled water group, mineral water group, and negative control, respectively) decreased after bathing; however, the decrease was not statistically significant (p=0.129; Fig. 4D).
After induction of psoriasis-like skin lesions with imiquimod, the spleen was enlarged as compared with that in normal mice. All populations of helper T cells, including Th1, Th2, Th17, and Treg cells, were increased after imiquimod treatment. However, after bathing with either distilled or mineral water, normal T cell proportions were observed. The proportion of Th1 cells increased in imiquimod treatment group (3.26%±1.3%); however in both distilled water bath group (1.86%±0.05%) and mineral water bath group (1.89%±0.22%), the proportion of Th1 cells decreased to the level of the negative group (1.87%±0.03%). This trend was also observed for Th2 and Th17 cells, which were increased after imiquimoid treatment to 2.72%±0.66% and 4.18%±0.92%, respectively. After bathing, the proportion of Th2 (1.23%±0.18% and 0.99%±0.14% in the distilled water and mineral water groups, respectively) and Th17 (2.16%±0.26% and 1.69%±0.21% in the distilled water and mineral water groups, respectively) cells was lower than the negative control (1.31%±0.13% for Th2 cells and 1.97%±0.11% for Th17 cells). Interestingly, the proportion of Foxp3+ regulatory T cells in the mineral water group (4.20%±0.07%) was higher than the distilled water group (2.50%±0.31%) and the negative control (1.56%±0.39%). Therefore, the proportion of Foxp3+ regulatory T cells was significantly lower in the distilled water group and in negative control (p=0.019) than in the imiquimod-treated group (positive control, 7.84%±1.58%); however, this statistically significant difference was not observed in the mineral water group (Fig. 5).
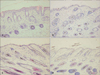
Histological evaluation of dorsal skin samples showed inflammatory cell infiltration and epidermal hyperplasia in the imiquimod-treated group (Fig. 6A). After 2 weeks of balneotherapy, epidermal hyperplasia was not observed in either the distilled water or mineral water group (Fig. 6B, C). Epidermal thickness, measured by optical microscopy, was significantly reduced in both the distilled water and mineral water groups (p<0.001; Fig. 7).
A history of the therapeutic use of thermal mineral water can be found throughout the world12,13,14,15,16,17,18,19,20,21. The chemical and physical composition of thermomineral waters vary depending on the geographical characteristics. The Dead Sea is a representative hypertonic mineral water that has a salt content of approximately 320 g/L. The major components of Dead Sea water include magnesium, calcium, potassium, and bromine22. Avene and La Roch-Posay thermal waters are well known low mineral waters that have a total dissolved solid content below 1 g/L. Although Avene thermal water is classified in the category of waters with low mineral content, the calcium, magnesium bicarbonate, and silicates in this water are thought to have immunomodulatory and anti-inflammatory properties23.
In the present study, the immunomodulatory effects of balneotherapy were evaluated using an imiquimod-induced psoriasis-like murine model. Lesional cytokines IL-17 and IL-23 were substantially lower in the mineral water group compared with the distilled water group. IL-23 plays a role in the pathogenesis of psoriasis by inducing the development of IL-17 and IL-22 producing Th17 cells. IL-17 and IL-23 play an important role in maintaining chronic inflammation in psoriasis24; thus, anti-IL-1725 and anti-IL-23 agents26 are considered treatment modalities for psoriasis. The inhibitory effects of balneotherapy on IL-17 and IL-23 expression explain the effectiveness of balneotherapy in relief from psoriasis. The level of IL-22, which mediates epidermal hyperplasia, was decreased in both the mineral water and distilled water groups. Decreasing levels of IL-22 was consistent with the decreased epidermal thickness observed in both the mineral water and distilled water groups. This result suggests that bathing alone removes scales from the skin and reduces inflammation.
Serum levels of IL-4 and IL-5 increased after imiquimod application and significantly decreased in the mineral water group but not the distilled water group after balneotherapy. This suggests that balneotherapy with mineral water can be effective for treatment of diseases characterized by polarization of Th2 cells, such as atopic dermatitis. The effects of mineral water on cytokines produced by Th2 cells are immunomodulatory and anti-inflammatory, as demonstrated by reduced levels of IL-423,27,28.
T cell proportions in the spleen also showed the beneficial effects of bathing. The proportions of Th1, Th2, and Th17 cells were normalized after bathing with both mineral water and distilled water. The proportion of Foxp3+ regulatory T cells was different between the mineral water and distilled water groups. Foxp3+ regulatory T cells inhibit inflammation; therefore, the elevated proportion of Foxp3+ regulatory T cells in the mineral water group could play a key role in the immunomodulatory effects of mineral water. Lower levels of IL-17 in the mineral water group might have resulted from increased proportion of Foxp3+ regulatory T cells, which are known to suppress Th17 cells. In addition to suppressive effects on Th17 cells, Foxp3+ regulatory T cells also suppress Th1 and Th2 immune responses by producing inhibitory cytokines, including IL-10 and transforming growth factor-β29. It is also possible that the moisturizing and thermal effects of bathing are responsible for reduced inflammation9.
The elements and their ideal concentrations or ratios essential for treatment of psoriasis with balneotherapy is yet to be ascertained. Several elements have been suggested to have immunomodulatory effects, including sulfur30, magnesium31, lithium31, bromine32, and selenium. The components of Hae-Un-Dae hot spring water showed low levels of magnesium, selenium, lithium, and other elements. The water from this hot spring was sulfurous saline thermal water with a total sulfur content of 77.3 mg/L. Sulfur has been shown to inhibit the proliferation of T lymphocytes30. Sulfur also has antibacterial and antifungal activities and keratolytic effects22. It is likely that the minerals in thermal waters exert immunomodulatory effects although is uncertain whether an undetected element or a combination of minerals might be important for these effects.
Several previous studies have reported the immunomodulatory effects of mineral waters. Thermal water has been shown to modulate cell membrane fluidity33 and reduce lipid peroxidation23. A previous study also showed that mineral water inhibits lymphocyte proliferation induced by epidermal Langerhans cells19. Thermal water has been shown to inhibit inflammatory cytokines IL-6 and IL-8, and this is considered a possible mechanism for psoriasis treatment34,35. The inhibitory effects of thermal water on endothelial cells have been studied. Thermal water was shown to inhibit the expression of vascular endothelial growth factor-A and to reduce inflammatory cell chemotaxis36. Thermal water also mediates the inhibition of tumor necrosis factor-induced E-selectin and intercellular adhesion molecule-1 expression in human endothelial cells37. Another study demonstrated the anti-inflammatory effects of mineral water by demonstrating a reduction in vasoactive intestinal peptide-induced inflammation, which seems to be at least in part related to alteration of the cutaneous neuropeptidergic system38. The findings can further explain the beneficial effects of balneotherapy in psoriasis.
There are several limitations to the present study. First, the number of mice in each study group (n=3) was too small to find a statistical significance between the study groups with respect to the clinical improvement scores. Second, spontaneous recovery was not investigated in this study. Despite these limitations, the present study provides novel data to demonstrate the trend of the anti-inflammatory activity and effectiveness of mineral water for the treatment of psoriasis.
In our previous study, the immunomodulatory effects of thermomineral water were shown to attenuate the differentiation of CD4+ T cells to Th1, Th2, and Th17 cells28. In the present study, key cytokines and T cells involved in the pathogenesis of psoriasis were modulated by balneotherapy with thermal mineral water. Taken together, these results suggest that balneotherapy can be potentially used as a beneficial treatment for psoriasis. Many patients with psoriasis request alternative therapies39, and balneotherapy can be an acceptable choice. Further studies are warranted to define the active ingredients of mineral water and the ratios of minerals required to reveal the mechanisms underlying this immunomodulatory activity. In addition, the mechanisms underlying the effects of Foxp3+ regulatory T cells need to be further elucidated.
Figures and Tables
Fig. 1
Clinical improvement of imiquimod-induced psoriasis-like skin lesions during 2 weeks of balneotherapy with distilled water or Hae-Un-Dae hot spring mineral water. IMQ: imiquimod, DW: distilled water, MW: mineral water.

Fig. 2
Clinical improvement scores for skin erythema (A) and scales (B). IMQ: imiquimod, DW: distilled water, MW: mineral water.

Fig. 3
Expressions of IL-17A, IL-23, and IL-22 mRNA in the skin. IMQ: imiquimod, DW: distilled water, MW: mineral water, IL: interleukin.

Fig. 4
Serum levels of IFN-γ (A), IL-4 (B), IL-5 (C), and IL-17A (D). IMQ: imiquimod, DW: distilled water, MW: mineral water, INF: interferon, IL: interleukin. *p<0.05; bar: standard error.

Fig. 5
Fluorescence-activated Cell Sorter analysis of splenic T cell profiles. Th1, Th2, Th17, and Foxp3+ Treg. IMQ: imiquimod, DW: distilled water, MW: mineral water. *p<0.05; bar: standard error.

Fig. 6
Histological evaluation and comparison of epidermal thickness (H&E, ×100). (A) Imiquimod-induced skin lesion, (B) group treated with distilled water, (C) group treated with mineral water, and (D) negative control group treated with vaseline.
References
1. Halevy S, Sukenik S. Different modalities of spa therapy for skin diseases at the Dead Sea area. Arch Dermatol. 1998; 134:1416–1420.

3. Langenbruch AK, Radtke MA, Augustin M. Quality of psoriasis care from the patients' perspective--results of the national health care study PsoReal. Eur J Dermatol. 2012; 22:518–524.

4. Harari M, Czarnowicki T, Fluss R, Ruzicka T, Ingber A. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012; 26:554–559.

5. Kudish AI, Harari M, Evseev EG. The measurement and analysis of normal incidence solar UVB radiation and its application to the photoclimatherapy protocol for psoriasis at the Dead Sea, Israel. Photochem Photobiol. 2011; 87:215–222.

6. Klein A, Schiffner R, Schiffner-Rohe J, Einsele-Krämer B, Heinlin J, Stolz W, et al. A randomized clinical trial in psoriasis: synchronous balneophototherapy with bathing in Dead Sea salt solution plus narrowband UVB vs. narrowband UVB alone (TOMESA-study group). J Eur Acad Dermatol Venereol. 2011; 25:570–578.

7. Peroni A, Gisondi P, Zanoni M, Girolomoni G. Balneotherapy for chronic plaque psoriasis at Comano spa in Trentino, Italy. Dermatol Ther. 2008; 21:Suppl 1. S31–S38.

8. Tabolli S, Calza A, Di Pietro C, Sampogna F, Abeni D. Quality of life of psoriasis patients before and after balneo -- or balneophototherapy. Yonsei Med J. 2009; 50:215–221.

9. Cozzi F, Lazzarin P, Todesco S, Cima L. Hypothalamic-pituitary-adrenal axis dysregulation in healthy subjects undergoing mud-bath applications. Arthritis Rheum. 1995; 38:724–726.

10. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836–5845.

11. Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, et al. Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ. 2007; 385:272–283.

21. Benedetto AV, Millikan LE. Mineral water and spas in the United States. Clin Dermatol. 1996; 14:583–600.

23. Merial-Kieny C, Castex-Rizzi N, Selas B, Mery S, Guerrero D. Avène Thermal Spring Water: an active component with specific properties. J Eur Acad Dermatol Venereol. 2011; 25:Suppl 1. 2–5.

24. Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006; 176:1908–1915.

25. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Psoriasis Study Group. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010; 2:52ra72.

26. Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J. ABT-874 Psoriasis Study Investigators. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008; 144:200–207.

27. Portalès P, Ariès MF, Licu D, Pinton J, Hernandez-Pion C, Gall Y, et al. Immunomodulation induced by Avène spring water on Th1- and Th2-dependent cytokine production in healthy subjects and atopic dermatitis patients. Skin Pharmacol Appl Skin Physiol. 2001; 14:234–242.

28. Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, et al. Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4(+) T cells. Ann Dermatol. 2012; 24:324–336.

29. Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008; 9:239–244.

30. Valitutti S, Castellino F, Musiani P. Effect of sulfurous (thermal) water on T lymphocyte proliferative response. Ann Allergy. 1990; 65:463–468.
31. Halevy S, Giryes H, Friger M, Grossman N, Karpas Z, Sarov B, et al. The role of trace elements in psoriatic patients undergoing balneotherapy with Dead Sea bath salt. Isr Med Assoc J. 2001; 3:828–832.
32. Shani J, Barak S, Ram M, Levi D, Pfeifer Y, Schlesinger T, et al. Serum bromine levels in psoriasis. Pharmacology. 1982; 25:297–307.

33. Cézanne L, Gaboriau F, Charveron M, Morlière P, Tocanne JF, Dubertret L. Effects of the Avène spring water on the dynamics of lipids in the membranes of cultured fibroblasts. Skin Pharmacol. 1993; 6:231–240.

34. Dal Pra I, Chiarini A, Pacchiana R, Zumiani G, Zanoni M, Armato U. Comano's (Trentino) thermal water interferes with tumour necrosis factor-alpha expression and interleukin-8 production and secretion by cultured human psoriatic keratinocytes: yet other mechanisms of its anti-psoriatic action. Int J Mol Med. 2007; 19:373–379.
35. Chiarini A, Dal Pra I, Pacchiana R, Zumiani G, Zanoni M, Armato U. Comano's (Trentino) thermal water interferes with interleukin-6 production and secretion and with cytokeratin-16 expression by cultured human psoriatic keratinocytes: further potential mechanisms of its anti-psoriatic action. Int J Mol Med. 2006; 18:1073–1079.

36. Chiarini A, Dal Pra I, Pacchiana R, Menapace L, Zumiani G, Zanoni M, et al. Comano's (Trentino) thermal water interferes with the expression and secretion of vascular endothelial growth factor-A protein isoforms by cultured human psoriatic keratinocytes: a potential mechanism of its anti-psoriatic action. Int J Mol Med. 2006; 18:17–25.

37. Castex-Rizzi N, Charveron M, Merial-Kieny C. Inhibition of TNF-alpha induced-adhesion molecules by Avène Thermal Spring Water in human endothelial cells. J Eur Acad Dermatol Venereol. 2011; 25:Suppl 1. 6–11.

38. Boisnic S, Branchet-Gumila MC, Segard C. Inhibitory effect of Avene spring water on vasoactive intestinal peptide-induced inflammation in surviving human skin. Int J Tissue React. 2001; 23:89–95.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download